Tiny metal nanoparticles are used as catalysts in many reactions, from chemical purification to the production of polymers and biofuels. The effectiveness of the activity of these catalysts in reactions such as these depends on the crystal faces exposed to the reaction
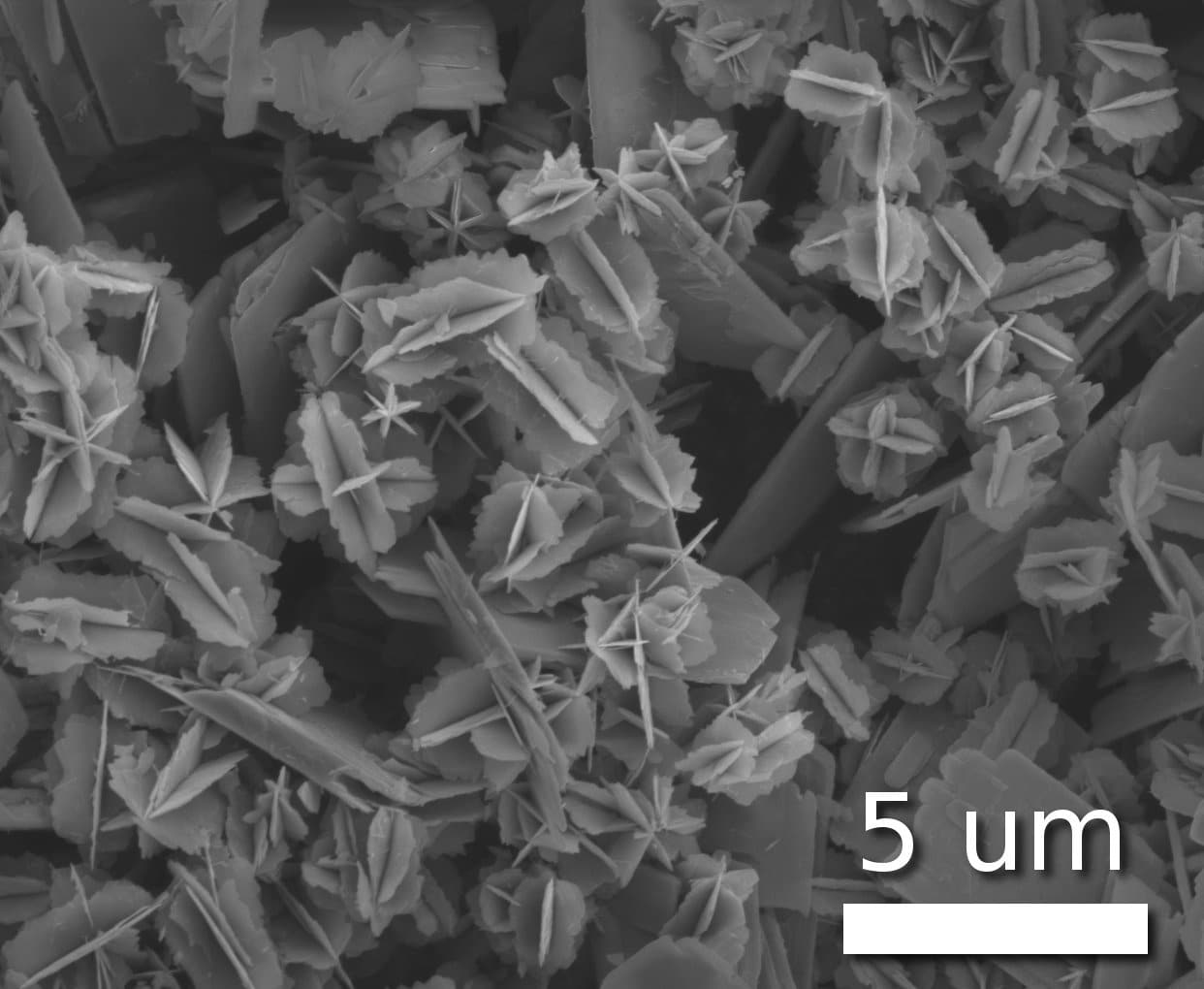
Tiny metal nanoparticles are used as catalysts in many reactions, from chemical purification to the production of polymers and biofuels. The effectiveness of the activity of these catalysts in reactions such as these depends on the crystal faces exposed to the reaction.
However, previous attempts to design these nanoparticles by changing their shape have failed since the structures are unstable and return to their original shape.
Now, researchers from Northwestern University's Energy Processing Catalysis Institute have discovered a new method for preparing metal nanoparticles for catalysis that promises to increase selectivity and utilization for a wide range of structure-sensitive catalytic reactions. The team, led by researcher Laurence D. Marks, professor of materials science and engineering, discovered that nanoparticles can be shaped using the particle's supporting structure.
"Instead of trying to engineer the nanoparticles, we engineered the substrate on which the nanoparticle is placed," said the lead researcher. "It changes the type of wigs that are exposed." The findings were published in the journal Nano Letters.
This solution was a kind of discovery: the team that prepared the nanoparticle samples discovered that it could not change their shape and turned to understand what exactly was happening. It turned out to the scientists that epitaxy - the spatial relationship between the position of the atoms of the nanoparticle and the position of the atoms of the substrate - was a more important factor in the design than previously thought.
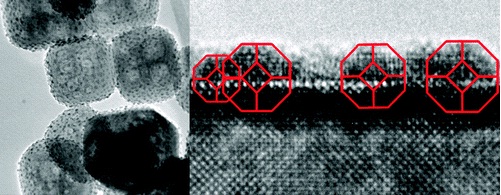
The team is currently testing the nanoparticles in a catalytic mop, and the initial findings look promising, the researcher notes. The nanoparticle appears to be stable enough to survive its long-term and strenuous activity as a catalyst.
"Our findings open the door to the development of better and more efficient catalysts," notes the researcher. "This method could be used for a variety of different metal nanoparticles. This is an innovative approach, and it may have a significant impact on the field."

One response
This article was already about two months ago and talked about the proper use of a substrate for atomic arrangement - nothing new