The goal of the two-year clinical study conducted throughout Europe was to evaluate the safety and effectiveness of the ongoing use of DREAMS. 44 patients took part in the study, where none of the patients had a case of death or thrombus (blood clot) following the use of the stent.
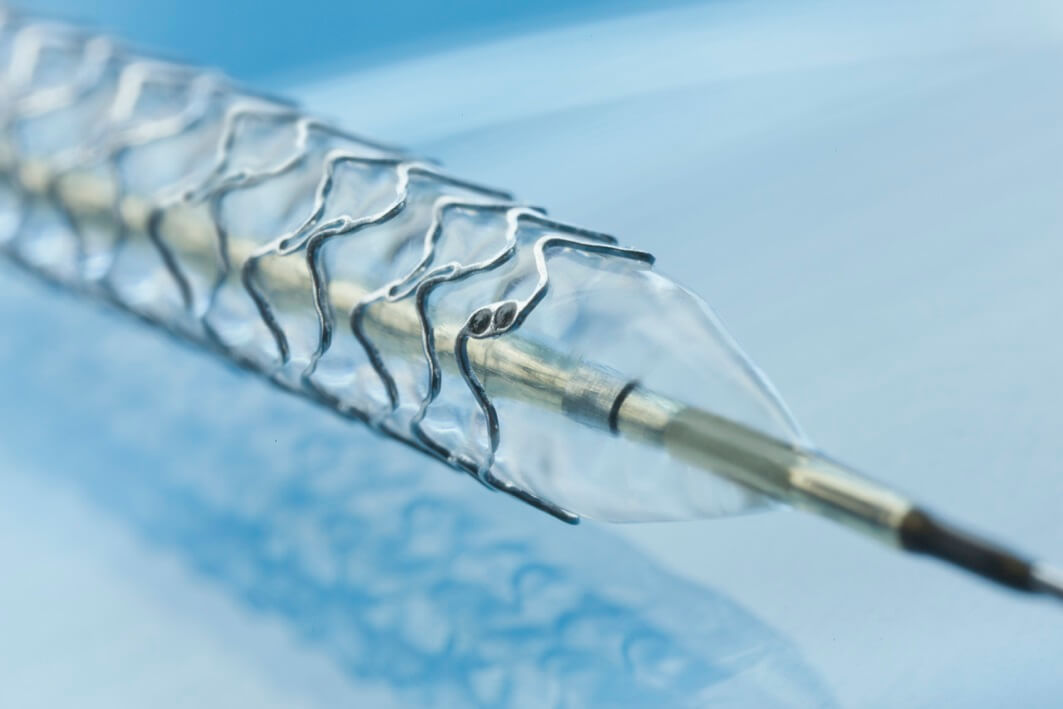
The results of the BIOSOLVE-I study, presented at the EuroPCR conference held in Paris, continue to demonstrate the high safety profile of using DREAMS - Drug Eluting Absorbable Metal Scaffold, an absorbable metal support that releases a drug, from Biotronic, intended for the treatment of coronary artery disease ).
The goal of the two-year clinical study conducted throughout Europe was to evaluate the safety and effectiveness of the ongoing use of DREAMS. 44 patients took part in the study, where none of the patients had a case of death or thrombus (blood clot) following the use of the stent.
Coronary artery disease is a condition in which fatty deposits form and accumulate on the walls of the coronary arteries that feed the heart muscle, constricting them and restricting blood flow. A heart attack is caused when the blood supply to a certain area of the heart muscle stops, for example, if a blood clot blocks one of the coronary arteries.
DREAMS is part of a highly innovative treatment option for patients with blockages in the arteries of the heart (as a result of coronary artery disease), developed by Biotronic, one of the world's leading manufacturers of developing, manufacturing and marketing medical devices for the heart. In contrast to the existing traditional treatment in which patients are treated - through the insertion of a permanent stent (support) that expands the damaged blood vessel, Biotronic's support is based on a magnesium skeleton, which is absorbed over time in the artery wall, leaving behind a restored blood vessel.
The restoration and reconstruction of the artery wall was also examined as part of the study. Through catheterization, which was performed in order to examine the twisting angle of the blood vessels and restore mobility, it was found that the blood vessels were indeed fully restored after a period of time of about a year from the day of catheterization. In fact, the blood vessel has already returned to normal movement function - vasomotion (contraction and expansion of the blood vessels) six months after implanting the stent. "Restoring vasomotion is one of the great advantages of absorbable supports," said Prof. Michael Haude, one of the researchers. "They help prevent the chronic pressure and inflammation on the arteries and enable a positive recovery of the damaged artery segment"
Based on the results of the current study, work on the design of the final product is currently complete and towards the end of the year it will enter the course of a large-scale clinical study.
