Compugen develops a system for cancer immunotherapy * Germany's Schott and Algatech establish an R&D site in Arava for the production of microalgae * Galamad will purchase systems from Itamar Medical for clinical research of people suffering from obesity and insulin resistance
Compugen is developing a system for cancer immunotherapy
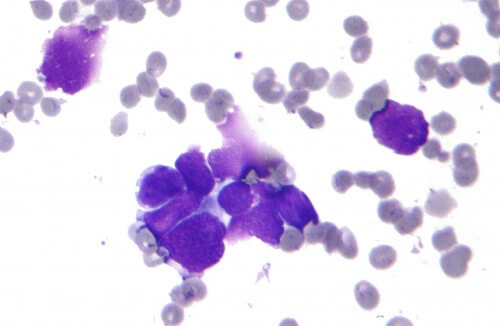
The new results support the putative role of CGEN-15049 as an immune checkpoint protein that inhibits the immune system's response against cancer cells
Compugen reported the results of additional trials, which continue to confirm that CGEN-15049 is a promising potential target for cancer immunotherapy. These latest experiments tested the effect of this protein, discovered by Compugen as having the potential to control the immune system, on immune system cells derived from cancerous tumors of melanoma patients. Based on these results and previous studies, the company continues to advance CGEN-15049, which is expressed on several types of cancer, including lung, ovarian, breast, colon, stomach, prostate and liver cancer, in the company's young drug pipeline, and is currently developing drug antibodies against a target This innovative therapeutic.
In the latest trials now reported, CGEN-15049 continued to show potential for inhibiting the immune system's ability to attack cancer cells. In fact, these experiments showed that increased expression of CGEN-15049 in human melanoma cells inhibits the anticancer activity of cytotoxic T cells derived from melanoma tumors taken from patients. These cells of the immune system, also known as tumor-infiltrating lymphocytes (TILs), penetrate into the tumor and play a major role in the immune response against the cancer cells. These results indicate that CGEN-15049 can inhibit the activity of the immune system in the tumor environment by inhibiting the TIL cells that are supposed to attack the tumor.
Additionally, preliminary data from a mouse cancer model continue to support that CGEN-15049 is expressed in cells of the immune system that suppress its activity in the tumor environment. These data, combined with the expression of this protein in a wide range of cancer types, as previously reported, and the effect of CGEN-15049 on the immune system cells involved in the response to the cancer tumor, support a possible role of this drug target in suppressing the immune system's responses against the cancer. In light of this, blocking the activity of CGEN-15049 by medicinal antibodies is expected to stimulate anti-cancer activity of the immune system, and lead to the disappearance of the tumor.
Success for the gene that will stop autoimmune diseases
Compugen today announced results of laboratory animal experiments supporting that a new mechanism of action is likely responsible for the long-term therapeutic response of CGEN-15001 in autoimmune diseases, presented in two sessions at the XNUMXrd International Conference on Immune Tolerance, held last week in Amsterdam, Netherlands.
The results are presented by Dr. Joseph R. Podujil, from the Feinberg School of Medicine at Northwestern University, in the USA, and by Dr. Iris Hecht, chief scientist in the company's project.
In a poster titled "Immune System Effects and Tolerability in a Multiple Sclerosis Model of CGEN-15001, a Fusion Protein Based on a Novel Immune Control Protein", Dr. Podujil describes results indicating that the long-term responses to CGEN-15001 treatment, demonstrated in laboratory animals, probably arise from the activity of regulatory T cells (Tregs), which play essential roles in maintaining immune tolerance. These preliminary results were recently obtained as part of Compugen's collaboration with Professor Steven D. Miller, Professor of Microbiology and Immunology at the Feinberg School of Medicine at Northwestern University in the USA. Dr. Podujil is a research professor in Professor Miller's lab.
The German company Schott and Algatech are setting up an R&D site in Arava for the production of microalgae
Algatech in Kibbutz Ketura will assign a beta site for the examination and open of innovative technological components to increase efficiency in the production of microalgae. This is the first collaboration of its kind in Israel for the giant Schott corporation.
The German company Schott is one of the oldest companies in the world in the field of glass. The company develops and manufactures modules of advanced systems for a variety of uses and is a leader in the development of glass components for the pharmaceutical, chemical and solar industries.
Algatech of Kibbutz Ketura is a world leader in the cultivation of microalgae for the production of unique natural ingredients for the pharmaceutical industry, nutritional supplements and cosmetics. Algatek recently announced that it will invest 60 million shekels in expanding its factory, in the development and production of new algae and in improving the processing processes of its flagship product: AstaPure - natural astaxanthin.
"We are very proud to cooperate and be a center for innovation for one of the leading companies in the world," said Hagai Stadler, CEO of Algatech. "Algatec's extensive knowledge in the field of algae cultivation technologies, combined with Schott's diverse production capabilities, will allow both companies to develop advanced solutions for the algae industry, and to expand our development capabilities in the field of microalgae-based products, a market that has been developing strongly in recent years all over the world, and as part of the trend the world for the consumption of food supplements and natural cosmetic products. Strengthening our production capabilities, and the production capabilities of high quality products with innovative technologies, will allow us to maintain our position as leaders in the global market."
Aviad Levy, director of the Israeli office of the Schott company: "The Schott company is very happy for the opportunity to work and step forward with a young and dynamic company that shows courage and a desire to lead the field of algae cultivation, which proves once again that even in Israel it is possible to have manufacturing companies with innovative technology capable of leading the market the world."
Glamad will purchase 60 EndoPAT systems from Itamar Medical as part of a clinical trial for those suffering from insulin resistance
Itamar Medical announced today that it has signed an agreement for the sale of systems and cooperation with the Glamed company, for the use of Itamar Medical's EndoPAT technology and systems as part of a large-scale clinical study conducted by Glamed. The clinical trial (phase b2) will start soon, with the participation of 240 NASH patients suffering from obesity and insulin resistance. The experiment will be conducted in 60 medical centers in 15 countries in Europe, Israel and Latin America. 14 central hospitals in Israel will participate in the experiment. EndoPAT systems will be installed in all these centers.
Glamad has developed an oral medication that is administered once a day to treat liver diseases and cholesterol-based gallstones. The drug he developed, Aramchol, is given to patients with fatty liver disease (NASH) who also suffer from obesity and insulin resistance. These patients are exposed to the highest risk of the two types of severe complications of the disease: cardiac problems and severe liver diseases, first cirrhosis of the liver and then liver cancer, which may require a liver transplant.
Itamar Medical's EndoPAT systems for testing arterial function will be integrated into the clinical trial planned by Galamad. The purpose of the experiment is to prove that the drug Armachol succeeds in reducing the accumulation of fat in the liver, thereby reducing or completely suppressing the inflammatory process that leads to the fatal liver complications and an increase in cardio-metabolic risks.
Alan Beharev, CEO of Galamad, responded that: "Epidemiological studies show that over 30% of the population suffers from fatty liver. It is estimated that about a quarter of them suffer from fatty liver inflammation - that is, about 55 million people in the US and the 5 largest European countries suffer from NASH - a disease in which the fat in the liver turns into inflammatory tissue, and these patients are exposed to the highest risk of the two types of severe complications of the disease : Cardio-metabolic problems and liver diseases when the inflammation develops into fibrosis (scarring of the liver) and then to cirrhosis of the liver and liver cancer which may require a liver transplant. As part of the upcoming experiment, we intend to examine the effect of the drug we developed on the disappearance of inflammation in the liver, as well as the improvement in endothelial function (arterial function), which is an important marker for cardiovascular events after 52 weeks of treatment."
Gilad Glick, CEO of Itamar Medical, said: "This is an important expression of confidence and further evidence of the great interest and need in the EndoPAT technology for diagnosing arterial function from a promising Israeli pharmaceutical company traded on Nasdaq. We are proud of this collaboration of two Israeli companies that are innovative in their field and see importance in the fact that a company developing a drug with great potential has chosen to use Itamar Medical's technology as a marker for cardiovascular risk within a large-scale clinical trial. This experiment joins other experiments that have been going on for several years in collaboration with leading pharmaceutical companies such as Roche and AstraZeneca".
Glamad will purchase 60 EndoPAT™ systems from Itamar Medical in order to integrate the arterial function test as part of a large-scale clinical trial that the company is conducting with the participation of approximately 240 NASH patients suffering from obesity and insulin resistance
Itamar Medical announced today that it has signed an agreement for the sale of systems and cooperation with the Glamed company, for the use of Itamar Medical's EndoPAT™ technology and systems as part of a large-scale clinical study conducted by Glamed. The clinical trial (phase b2) will start soon, with the participation of 240 NASH patients suffering from obesity and insulin resistance. The experiment will be conducted in 60 medical centers in 15 countries in Europe, Israel and Latin America. 14 central hospitals in Israel will participate in the experiment. EndoPAT systems will be installed in all these centers.
Glamad has developed an oral medication that is administered once a day to treat liver diseases and cholesterol-based gallstones. The drug he developed, Aramchol, is given to patients with fatty liver disease (NASH) who also suffer from obesity and insulin resistance. These patients are exposed to the highest risk of the two types of severe complications of the disease: cardiac problems and severe liver diseases, first cirrhosis of the liver and then liver cancer, which may require a liver transplant.
Itamar Medical's EndoPAT systems for testing arterial function will be integrated into the clinical trial planned by Galamad. The purpose of the experiment is to prove that the drug Armachol succeeds in reducing the accumulation of fat in the liver, thereby reducing or completely suppressing the inflammatory process that leads to the fatal liver complications and an increase in cardio-metabolic risks.
Alan Beharev, CEO of Galamad, responded that: "Epidemiological studies show that over 30% of the population suffers from fatty liver. It is estimated that about a quarter of them suffer from fatty liver inflammation - that is, about 55 million people in the US and the 5 largest European countries suffer from NASH - a disease in which the fat in the liver turns into inflammatory tissue, and these patients are exposed to the highest risk of the two types of severe complications of the disease : Cardio-metabolic problems and liver diseases when the inflammation develops into fibrosis (scarring of the liver) and then to cirrhosis of the liver and liver cancer which may require a liver transplant. As part of the upcoming experiment, we intend to examine the effect of the drug we developed on the disappearance of inflammation in the liver, as well as the improvement in endothelial function (arterial function), which is an important marker for cardiovascular events after 52 weeks of treatment."
Gilad Glick, CEO of Itamar Medical, said: "This is an important expression of confidence and further evidence of the great interest and need in the EndoPAT technology for diagnosing arterial function from a promising Israeli pharmaceutical company traded on Nasdaq. We are proud of this collaboration of two Israeli companies that are innovative in their field and see importance in the fact that a company developing a drug with great potential has chosen to use Itamar Medical's technology as a marker for cardiovascular risk within a large-scale clinical trial. This experiment joins other experiments that have been going on for several years in collaboration with leading pharmaceutical companies such as Roche and AstraZeneca".

One response
Can someone please provide a link to the biomed company index in Israel?