The Bioboost Group, owned by the Orbimed Foundation and the multinational corporations Johnson & Johnson and TAKEDA, won the concession to establish the new biotechnological incubator and operate it for a period of 8 years

Chief Scientist at the Ministry of Economy Avi Hasson: "The new biotechnology incubator is part of the government's strategic activity to strengthen and establish the biotechnology industry in Israel. This is a golden opportunity to establish companies here in Israel that will remain for the long term."
At the end of a competitive process in which several groups competed, today the Biotechnological Incubator Committee headed by the Chief Scientist of the Ministry of Economy, Avi Hasson, chose the proposal of the Bioboost Group as the winning proposal to receive the concession for the establishment of the new Biotechnological Incubator and its operation in the science park in Ness Ziona, for a period of 8 years. The new biotechnological incubator will work to encourage technological entrepreneurship in the fields of biopharma as well as the industrial application of technologies in these fields, from the research and development stage through the phase of proving the feasibility to clinical trials, while cooperating between the research institutions in Israel, and between the biotechnological incubator and the multinational corporations involved in it. The winning group has a unique composition of shareholders that includes two pharmaceutical companies, one of the leading in the world, together with a leading venture capital fund, in the field of drug development and biotechnology.
Projects accepted into the incubator will be entitled to a government grant of up to NIS 6.9 million for 3 years, which is 85% of the total budget approved for the project. The remaining 15% will be invested by the greenhouse itself. The government grant to the state will be returned to the state by the company only in case of success, through royalties from revenues received from the sale of the developed product. The incubator will also be entitled to take in, under certain conditions, other ventures from the field of life sciences such as medical devices, but the main investments will be in the establishment of new biopharma companies.
The Chief Scientist of the Ministry of Economy, Avi Hasson: "The new biotechnological incubator is an important pillar in the activities of the Office of the Chief Scientist to position Israel as a world leader in drug development." Hasson points out that a lot of research activity in the field of biopharma is carried out in research institutions throughout the country, and there are private entrepreneurs with brilliant ideas that are groundbreaking in the field, but there is a market failure in bringing these studies to general industrial application and this is due to the private market's reluctance to invest in the early stages of seed. Hasson explains that this is the place of the biotechnological incubator and through it it will be possible to realize the great potential that is waiting to be realized. Hasson adds that the fact that a group was chosen that brings together all the relevant disciplines and that runs long distances, will not only ensure the establishment of many new biopharma companies in Israel, but will also accompany them further down the road many years after they have finished the incubation period. This is a golden opportunity, Hasson says, to establish companies here in Israel that will remain for the long term. Hasson adds that "the new biotechnology incubator is part of the government's strategic activity to strengthen and establish the biotechnology industry in Israel." Hasson also added that the Office of the Chief Scientist sees great importance in both the creation of new tools and the necessary reforms in existing tools, in order to improve the quality of support for research and development in Israel, and the new biotechnological incubator is just one of many examples of this.
The director of the technology incubator program in the Office of the Chief Scientist in the Office of the College, Yossi Smoller emphasizes that "a combination of the world's leading pharmaceutical corporations (sales turnover of over 5 billion dollars per year) should significantly increase the chances of success of the incubator companies, both due to the large financial resources available to corporations These, and they are due to the accessibility they will allow the incubator companies to technological personnel, to the physical infrastructures they have, to opinion leaders, and to their customer base.
The deputy in charge of budgets at the Treasury, Yonatan Regev, noted that the harnessing of giant companies such as Johnson & Johnson and Takeda, whose first entry into investments in Israel, to direct and deep involvement in the startup scene in Israel, is a great achievement for the biotechnology industry in Israel and is expected to contribute greatly to the Israeli economy.
The Silantis company from the Technion will market in Europe the vascular seal it developed
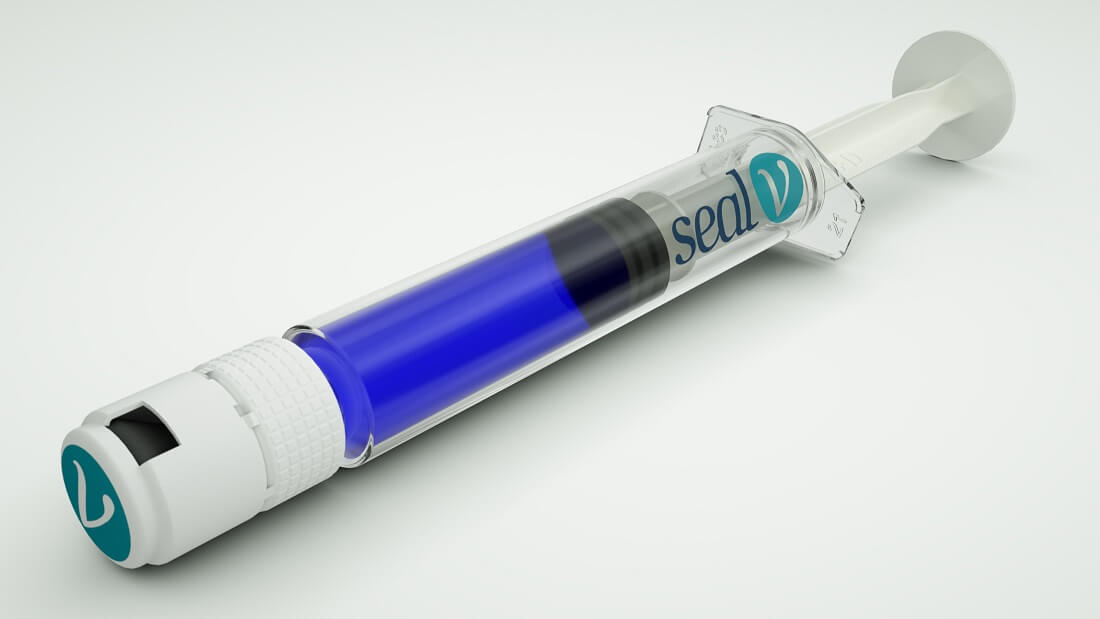
The Seal-VTM seal was developed by the company following the research of Professor Havaslat Bianco-Feld from the Technion. It is designed to stop bleeding from suture lines in large blood vessels
Silantis, a private start-up company developing innovative tissue adhesives that mimic underwater algae adhesion, reports that it has received CE approval for European marketing of the Seal-VTM vascular seal.
Seal-VTM is a protein-free sealant designed to stop bleeding by mechanically sealing potential leak areas from surgical connections of large vessels, such as the carotid artery (carotid), femoral artery, brachial artery, and iliac artery (pelvis).
Seal-VTM, based on the imitation of the adhesion mechanism of algae under water, heralds a new generation in tissue adhesion. The product has an inherent ability to adhere firmly to natural and synthetic blood vessels even in a wet or humid environment. The protein-free composition of the gasket prevents risks associated with the use of protein-based products. Also, unlike hemostatic agents, which work by activating the coagulation system, Seal-VTM does not need the presence of blood in order to work effectively, so it can be used as a prophylactic seal and prevent bleeding.
"We are excited about receiving approval to market Seal-VTM in the European market, and are happy to offer surgeons a new and better alternative to control bleeding from surgical suture lines," says Tomer Fox, CEO of Sealantis. Fox joined Silentis recently, after holding senior management positions for two decades at global companies, including Medtronic Ventur, Vichy and Anord.
The chairman of Silantis, Dr. Ze'ev Gilkis, explains that "Silantis has worked closely with surgeons in order to leverage the extraordinary capabilities of its adhesion technology, and to develop products that will be well positioned in the tissue adhesion market, which is valued at billions of dollars."
In addition to Seal-VTM, Silantis develops a series of advanced solutions for a variety of medical needs, including an intestinal seal designed to prevent the leakage of intestinal contents from sutures in the digestive system - one of the fatal complications in intestinal surgeries; Adhesive to prevent swelling after aesthetic surgeries, which can significantly reduce the duration of recovery from facial and body stretching; and adhesives combined with drugs, for the targeted release of a drug at a specific internal site.
Sealantis is currently working on strategic partnerships for the intended launch of Seal-VTM in Europe.
Compugen signed a cooperation agreement in the field of cancer with the pharmaceutical giant Bayer
The license and collaboration agreement includes the research, development and commercialization of cancer immunotherapy against two new proteins discovered by Compugen
Compuage announced the signing of a licensing and collaboration agreement with Bayer HealthCare (Bayer) for the research, development and commercialization of cancer drugs based on antibodies against two new proteins discovered by Compuage that regulate the immune system. According to the agreement, Bayer and Compugen will jointly manage a preclinical research program. After that, Bayer will have full control over further development as well as global commercialization rights for potential cancer drugs.
Under the terms of the agreement, Compugen will receive an initial payment of $10 million, and is entitled to receive over $500 million in payments for potential milestones of the two development programs, not including milestone payments of up to $30 million related to preclinical activity . In addition, Compugen is entitled to royalties at a single-digit rate, medium to high, on global net sales of any product that will be developed as part of the collaboration.
"Bayer is committed to translating the science behind cancer research into effective medicines that will help people with cancer live longer and better quality lives," said Prof. Andreas Busch, member of the Bayer HealthCare Executive Committee and head of the Global Drug Discovery Program. "Antibody-based immunotherapy is a promising approach in oncology that can stimulate the body's immune cells to fight cancer cells. Immunotherapy is one of our four focus areas in oncology. We look forward to expanding our portfolio in this area following the collaboration with Compugen."
Dr. Anat Cohen-Deig, President and CEO of Compugen, added, "We are very excited to begin the collaboration with Bayer, a leading pharmaceutical company, with growing activity in the field of oncology. The collaboration focuses on the development of immunotherapeutic treatments for cancer based on antibodies against two promising protein targets that regulate the activity of the immune system. In addition, we believe that the prediction and validation of these two goals, with the help of our computational prediction infrastructures that are suitable for many and varied applications, give further validity to our long-standing commitment to establishing Compugen's unique capabilities."
Immunotherapy is an approach that aims to fight cancer by stimulating the body's own immune system cells to attack the tumor. The cancerous tumor and its environment suppress the ability of patients to develop an effective immune response against the tumor, and therefore enable the tumor's growth and survival. Compugen has discovered two new proteins that regulate the activity of the immune system and that may play a key role in suppressing the immune system. Compugen researchers are developing specific medicinal antibodies that aim to eliminate the suppression of the immune system by these proteins and thereby fight cancer by reactivating the immune response against the tumor.
Based on press releases from the Ministry of Economy, the Technion and Compugen
