All the news was provided by the companies to the stock exchange last week (30/12/2013-2/1/2014)
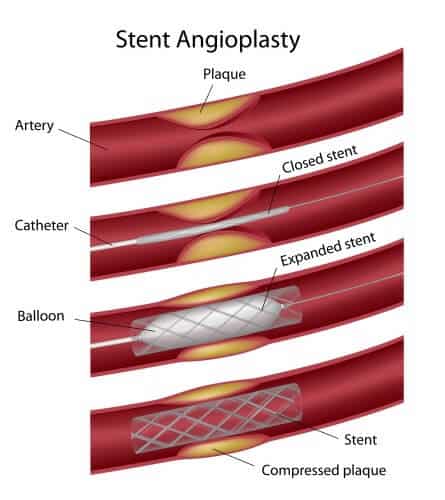
Biotronic Israel launches the Orsiro, a drug-eluting hybrid stent containing a biodegradable polymer. The launch came after receiving approval from the Israeli Ministry of Health.
The Orsiro is coated with a layer of biodegradable polymer - Drug Eluting Stent. The metallic stent is coated with a passive Probio layer that almost completely prevents the interaction between the stent and the surrounding tissues. The active coating, the Biolute, contains a biologically adapted polymer that releases the drug absorbed in the surrounding tissues. Over time, after the absorption of the drug and the polymer, the metal stent with the passive coating remains in the artery.
Ormed received permission to register a patent in Israel and Australia for technology for the oral administration of proteins
Ormed Pharmaceuticals, a pharmaceutical company in the clinical stage that focuses on developing a unique technology for oral administration of drugs that are currently given only by injection, announced today that it received approval for patent registration from the Israeli and Australian patent offices. The patent titled, "Methods and Compositions for Oral Administrations of Proteins", protects the company's core technology for oral administration of drugs and vaccines that are given today by injection.
Intech Pharma received approval for the accordion pill patent in Japan as well
The Intech Pharma company, which deals in improving medicines, reported that it received approval from the Japanese Patent Office to register a patent for the production method of the accordion pill. This patent expands and strengthens the intellectual property of Intech Pharma, which has completed and received to date similar patent approvals in the USA and Israel.
The patent refers to and protects the production method of the accordion pill platform which includes a process of folding and integrating various elements that make up the system. The patent will be valid until 2027.
Redhill receives investment from the Broadfin Foundation
The Israeli biopharmaceutical company, Redhill Biopharma, which develops patent-protected drugs in advanced clinical development stages, reports that it has signed an investment agreement with Broadfin Capital Broadfin for the sale of shares and options in a private offering for a total amount of 2.5 million dollars.
The proceeds of the private offering will be used for the company's general needs and to finance its research and development activities, including the continued development of the drugs for gastrointestinal diseases, RHB-104 for Crohn's disease and RHB-105 for H. pylori infection, which are currently in phase III clinical trials in the USA B.
Yes Pate analyzes the results of the clinical trial
The Yes Fit Biopharma group, which develops drugs for inflammatory diseases and cancer, announced today that a retrospective analysis of the phase 3 trial in dry eye of the Optlix subsidiary will be performed, based on the analysis of the level of the adenosine type 3A biomarker (which is the target of the 101CF drug) of the patients in the trial . This analysis is based on the positive results recently obtained in the phase 2b trial of the 101CF drug in rheumatoid arthritis patients, in which only patients with an adequate pre-treatment biomarker level were included. In order to perform the aforementioned analysis, blood tests will be taken from patients who participated in the experiment and the correlation between the biomarker levels and the patients' response to the treatment will be tested.
Two more orders for Mazur systems, a total of 7 systems were installed in the fourth quarter
Mazor Robotics, which develops and sells medical equipment for navigating surgical tools with high precision and with minimal invasiveness for spine surgeries, announces an order for two Renaissance systems at the end of December. With this, the company completes the sale of seven systems in the fourth quarter of 2013. In the US, one system was ordered by Littleton Adventist Hospital and the other system was ordered by Bestech, the distributor of the company's products in Israel, for installation at Bnei Zion Hospital in Haifa.
