Interview with Prof. Mishna Galia Mein, Head of the Biomimetic Chemistry Laboratory at the Technion / by: Yael Halfman Cohen

In recent years, a laboratory for biomimetic chemistry has been operating in the Faculty of Chemistry at the Technion, under the leadership of Associate Prof. Galia Ma'in. In the landscape of biomimetic innovation, which is today more identified with engineering developments at high levels of imitation such as imitation of organs or organisms and less identified with chemical developments, this laboratory, which deals with biomimetic imitation at the molecular level, stands out. It turns out that even at the molecular level there is research and imitation of functions and actions related to structures, and the result is innovation.
The laboratory deals with several biomimetic developments based on basic chemistry.
The first project deals with biomimetic enzymatic catalysis. The project is based on the study of foldamers, molecules that can fold in solution by forming non-covalent bonds. Foldamers can have a helical structure, for example, which mimics the helical structure present in natural polymers, such as peptides and proteins. This folded structure enables the activity of proteins, such as, for example, selective catalysis of reactions.
In the laboratory, a peptide-like molecule was developed, which organizes itself in the form of a helix (right or left). They attached a catalyst to it that knows how to oxidize alcohol. The catalyst is directionless. When attached to the coil, the catalyst receives a direction from the coil, and is able to selectively catalyze a right (or left) molecule and leave the left (or right) molecule in solution. This is how a separation is made between right and left kohls that are in the mixture.
Possible applications: in the synthesis processes of molecules, where separation between pairs of molecules is required and a catalyst is needed to separate them for various applications. In drug production processes, for example, molecules with a specific orientation are required (for example, only the molecule with the right orientation is the drug, while the one with the left orientation is inactive, or even harmful), while the synthesis process often produces mixtures, so separation is required.
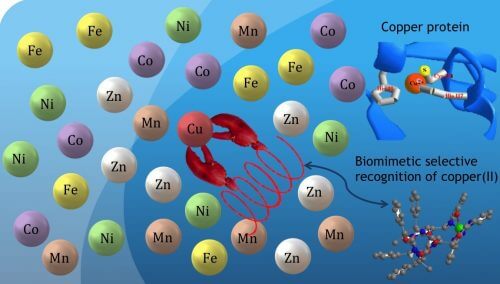
The second project deals with biomimetic selectivity. Different molecules in our bodies or in nature bind only to specific metal ions (eg copper ions) from the variety of metal ions (iron, zinc, magnesium, etc.) for their operation. In the laboratory they created a helix-like molecule, which has two units attached to the same side of the helix, which can capture only copper ions from the "sea" of metal ions (see Photo 1 which simulates the process). Future applications for this may be in the medical world. For example, diseases related to inadequate activity of the brain in old age, such as dementia, are associated with an excess accumulation of metal ions such as copper, zinc, iron or aluminum. Future development of biomimetic selectivity may allow selective capture of these ions and their removal from the body.
The third project deals with accelerating biomimetic reactions. The photosynthetic system that works in plants and algae knows how to produce energy from sunlight, and if we succeed in imitating it, we can deal with the problem of the ever-depleting energy. The system includes two parts: one part absorbs sunlight and water, oxidizes the water by solar energy, and produces oxygen, protons and electrons. The second part takes the protons and electrons and produces hydrogen and sugar from them (energy sources).
In the laboratory, a molecule was synthesized that is similar in its structure to a complex that contains manganese ions, which is a catalyst in the water oxidation process. The molecule was designed to be soluble in water, so that it would be possible to investigate whether it could catalyze their oxidation by a low electrical potential (an environmental advantage). Indeed, the molecule was found to be soluble and stable in water, showing the ability to produce more electrons than were invested in it alongside high levels of oxygen. Possible applications: production of hydrogen as a "green" and available substitute for gas as an energy source.
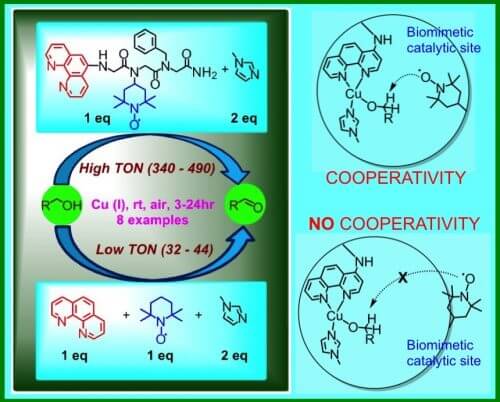
The fourth project deals with catalysts working in cooperatives. An enzyme can accelerate a process 600,000 times faster, and this at body temperature and atmospheric pressure of the living environment. For comparison, today in industry (for example: oxidation of alcohols, oxidation of methane to methanol, production of ammonia) catalysts are used that operate at a temperature of 500 degrees and at very high pressures.
It turns out that a prominent principle in the process of natural acceleration is the principle of cooperation. Each enzyme has at least one active site, the site where the process of catalyzing a specific reaction occurs. This site contains 2-3 amino acids, and often also a metal ion or ions. Although only one of the amino acids or the metal ion actually connects to the molecule that turned into another catalyzed molecule, the rest help in the process - for example, they stabilize the amino acid or the oxidation state of the metal ion.
In the laboratory, they imitated the sharing feature, and showed that if you take two different catalysts that work in solution in a cooperative way, and fix them on another molecule so that an "active site" is created, the cooperation increases greatly and the efficiency of the catalyzed reaction can be increased 20-25 times (see Photo 2 showing this process).
It seems that there is a lot of innovation potential at the molecular level, and that we will hear many more in the coming years about biomimetic chemistry.
The news was written following an interview with Associate Prof. Galia Mein, Head of the Biomimetic Chemistry Laboratory at the Technion.
