Weizmann Institute scientists have developed a method to measure the speed at which a single nanopore transfers electrical charge. The findings may help produce more efficient batteries

The nanopores increase the surface area for storing charged particles. Photo: Joe Monk, Wikimedia
Nomophobia (abbreviation of no-mobile-phone phobia) is defined as the fear of being left without cellular reception. Even if we don't suffer such an extreme reaction, our dependence on the longevity of rechargeable batteries is increasing day by day - whether it's smartphones, laptops, electric cars or many other devices. A common solution for efficient energy storage is the use of "supercapacitors" - power units that store charge in electrodes made of porous materials. The pores - tiny cavities and crevices - enormously increase the surface area for storing charged particles, but also slow down the rate at which the electrodes pick up an electrical charge and then remove it. As recently reported in the scientific journal Nature Communications, Weizmann Institute of Science scientists have developed a method to measure the speed at which a single nanopore transfers electrical charge. These findings may help in the development of more efficient porous materials for use in battery production.

When the scientists set out, batteries or supercapacitors were not even on their minds. Prof. Jacob Klein from the Department of Materials and Surfaces and his colleagues wanted to know what happens to the forces acting between two surfaces in the nanoscale when the electrical potential of one of them is changed. They created a device consisting of two extremely smooth round plates - one of gold and the other of a mineral insulating material called mica (or mica) - which were placed opposite each other at a minimal distance; This creates a nanogroove with a diameter of about 100 microns (millionths of a meter) and a width of about 50 nanometers (billionths of a meter). The scientists dipped the plates in a solution containing charged ions, suddenly changed the potential of the gold surface and followed the development of the electrostatic force - the force of repulsion or attraction between two charged surfaces.
"This is how we realized that measuring the forces acting in a nano-groove allows us to measure the charging duration of a nano-pore in the electrode - that is, how long it takes for ions carrying a charge to enter or leave the pore," says Prof. Klein, who conducted the research in collaboration with Dr. Ran Tevoni and Dr. Gilad Silbert, then research students in his group, and with his department colleague Prof. Shmuel Shafran and Prof. Philip Pincus from the University of California at Santa Barbara. The researchers relied on a previous study carried out by Dr. Liraz Chai, then a research student in Prof. Klein's laboratory.
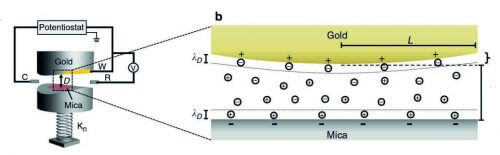
The scientists found that the time it took charge-carrying ions to enter or exit the nanoslot was about one second. It was also discovered that different characteristics affected the speed of entry and exit. For example, the charging was much faster if the nanogroove was wider and when the concentration of ions in the solution was greater. The findings indicate that these measurements may help in the development of more efficient porous materials for use in battery production. The measurements may also help improve porous materials for use in other technologies, such as water desalination or the production of renewable energy from salt solutions.
