An "arms race" is going on between the antibiotics and the bacteria, which manage to acquire resistance against them. who will win? - An article by Zvi Atzmon published in Galileo in 1994. More topical than ever
At the end of World War II, humanity was faced with a weapon that threatened to destroy it completely from the face of the earth - the atomic bomb. And to contrast: a few years earlier, during the same war, the disease-causing (pathogenic) bacteria were exposed to the antibiotic weapon. Many hoped that it would eliminate diseases such as bacterial pneumonia, bacterial meningitis, sepsis following childbirth, injury or surgery, tuberculosis, syphilis, gonorrhea, anything - diseases that have killed countless victims throughout history, and caused indescribable suffering.
But while the nuclear demon is still imprisoned and is only a threat, the genie - the resistance of the bacteria to antibiotics - has already slipped out of the antibiotic bottles. Indeed, today, more than fifty years since the sick and wounded were treated with penicillin for the first time, and when more than a hundred different antibiotic drugs are placed on the shelf ready for use, people are dying from diseases that were supposed to become a thing of history. And these are not backward areas: according to the report of the American Center for Disease Control and Prevention, in 1992 more than 13 thousand hospitalized patients died in the United States due to bacterial infections resistant to antibiotic drugs. And if this alarming number is not enough, the future looks even more alarming. In March of this year, an article was published in Newsweek, the title of which was "The End of Antibiotics" written in bold capital letters. In April, most of one of the issues of the prestigious scientific journal Science was devoted to the subject, but its title was more subdued: "Resistance to Antibiotics". First, we allowed ourselves to attach the term gene to the phenomenon of the bacteria's resistance to antibiotics, both to describe it as a dangerous demon that hatched from the medicine bottles, and as a linguistic refinement, implying that the origin of this demon is a hereditary change, a slight change of a gene.
Do not make the mistake of thinking that hospital bacteria are resistant, God forbid, to all antibiotic drugs - no, we have not yet reached the end of antibiotics - but today many strains are resistant to many of the drugs, and sometimes until the medical team manages to identify an effective drug against the bacteria, the patient's systems may suffer a fatal injury , and the situation is expected to worsen. Today, many of the strains of Staphylococcus aureus that infest hospitals are resistant to all types of antibiotics, except for one - vancomycin. It is clear to everyone that at some point one of the staphylococci will acquire resistance to vancomycin as well; when this last line of defense is breached, the doctors and patients will Helpless, like in the days before antibiotics.
unusual drugs
In 1929, Alexander Fleming reported on an observation he had made: colonies of staphylococci disappeared from the growing soil, or more precisely they passed through (Iysiis), when the mold fungus Penicillium developed near them. It took 11 years before Cheyenne, Flory and their colleagues were able to exhaust a significant amount
of penicillin from the mold fungus Penicillium notatum (P. Notaton). In 1941, penicillin began to be used for medical purposes, and already in World War II, the lives of many wounded people were saved thanks to the penicillin injections. In 1949, the pharmaceutical factories were already producing quantities that met market demands. Penicillin G has been found to be a very effective drug against many species of pathogenic bacteria, and its use usually does not involve negative side effects. However, the original penicillins, all of which were produced from mold fungi, had four disadvantages: their activity against gram-negative bacteria (see box) was low; they were sensitive to acids, and therefore could not be taken by ingestion because they were destroyed in the stomach; they provoked allergic reactions in some people (the problem) still known today (; they were broken down by the enzyme penicillinase. In order to overcome at least some of these shortcomings, Chein and his colleagues tried to extract the molecule common to the different penicillins and develop a better drug. Their efforts did bear fruit in 1957, and thus the path was opened to the creation of semi-artificial penicillins (semi-synthetic), including penicillins (such as ampicillin, amoxicillin), which can be taken by ingestion, being resistant to stomach acidity.
The penicillins belong to a group of drugs that damage the bacterial cell wall. The penicillin molecules bind to special proteins (called "penicillin-binding proteins") in the wall. These proteins play different roles in the building stages of the wall, and their attachment to the penicillin molecules prevents the completion of construction. The bacterial cell, whose mechanical shield is damaged, explodes due to osmotic absorption of water.
One of the effective defense mechanisms of the bacteria against penicillin is the creation of penicillinase - an enzyme that breaks down penicillins. Already in 1940, even before the medical use of penicillin began, Abraham and Chain identified an enzyme secreted by bacteria that breaks down penicillin. Although they hypothesized that the enzyme might sabotage the effectiveness of the medicinal use of penicillin, it can be assumed that even they did not know how right they were. Vancomycin, which we mentioned above, also kills bacteria by damaging the bacteria's cell wall, although it is not part of the penicillin group.
Many antibiotic drugs do not damage the bacterial cell wall but other sites and functions. There are drugs that harm the functions of the bacterial cell membrane, and there are drugs that harm the processes of building the nucleic acids; Many antibiotic drugs damage the protein building process.
To fight with a ribosome - to sabotage a protein
Below are several antibiotic drugs and their sites of action in the bacterial cell.
Chloramphenicol (known by its trade name Sintomycin) was first produced in 1947 from a species of Streptomyces bacterium. Already in 9491 they began to produce it synthetically. Chloramphenicol binds to the ribosomes in the bacterial cell - those organelles that serve as the "protein factories", thus inhibiting the production of proteins in the bacteria. Among the bacterial populations, one can find relatively resistant mutants to chloramphenicol, being less permeable to the drug; Apart from this, there are bacteria that produce an enzyme that performs a chemical change in the chloramphenicol molecule, thus canceling its action as a drug.
Aminoglycosides - a group of antibiotic drugs (among them: streptomycin, neomycin) that bind to the ribosomes of the bacterial cell and inhibit protein production. Over the years, bacteria have developed several mechanisms of resistance to aminoglycosides, such as mechanisms that prevent the drug from entering the bacterial cell; ribosome proteins that have no affinity for the drugs have evolved; an enzyme that performs a change chemical in the drug and thus cancels its activity Streptomycin was first isolated from a species of Streptomyces by Waxman and Schatz in 1944.
Tetracyclines - a group of drugs, the first representative of which was isolated in 1948 from a species of Streptomyces. After they enter the bacterial cell, the tetracyclines bind to the ribosomes and inhibit the construction of the proteins. The resistance to tetracyclines may arise due to their non-penetration into the cell wall, or the bacterium has an active removal mechanism (removal from the bacterium using an energy-consuming pump).
Macrolides - the best known drug from this group is erythromycin. It was first produced in 1952 from one of the Streptomyces species. The macrolides bind to ribosomes and prevent the construction of proteins in the bacterial cell. The resistance of bacteria to macrolides is a fairly common phenomenon, and originates from ribosomes whose components have no affinity for macrolides.
Durability - how?
The factors that give bacteria resistance (complete or low sensitivity to a drug) to a certain antibiotic drug are many and different. In many cases, bacteria whose rate of metabolism and reproduction are slow are more resistant to certain types of antibiotics. Such is, for example, the tuberculosis bacterium. In another case, in Gram-negative bacteria, the outer membrane covering the wall makes it difficult for certain substances, including drugs, to pass through.
The particularly interesting and threatening phenomenon is the resistance of the bacteria that were initially sensitive to the drug. This phenomenon is an example of an evolutionary process, the "fuel" that drives it is strong selective pressure (selection), which is created under the conditions of the use of antibiotic drugs. Since these are very rapid changes - after all, the entire history of the medical use of antibiotics does not exceed much more than 50 years - we can certainly say that we are facing evolution-in-eye, or: evolution in real time. It must be remembered that these are hereditary changes - the resistant population is genetically different from the original population, which is sensitive to the drug.
Many mechanisms may confer resistance to bacterial strains that were originally susceptible. One mechanism is the appearance of a gene that encodes the creation of an enzyme that acts on the drug, either by way of chemical decomposition, or by way of another chemical change, thereby canceling its effectiveness. Another mechanism is a change in the target molecule - the molecule of the bacteria that the drug affects. In this case, it is possible to distinguish between a change (mutation) in an existing gene, which codes for the creation of the molecule, and the "acquisition" of a gene that codes for the creation of a completely new enzyme in the cell, an enzyme that chemically changes the target molecule and makes it unrelated to antibiotics.
Another mechanism conferring resistance is changing the permeability properties of the membrane (of the cell membrane, or of the outer membrane in gram-negative bacteria), which makes it less permeable to the drug. Such a change cannot, usually, confer total resistance (because if it is small molecules, some permeability will remain in any case), but only in reducing the sensitivity of the bacterium to the drug.
A fourth option is the purchase of a mechanism to remove the drug from the cell with the help of a special protein located in the membrane that uses energy to actively remove the drug from the cytoplasm. From the beginning of the XNUMXs, the importance of active washing as a mechanism that confers resistance became clear. This follows the research of Stuart Levy and his colleagues from Tufts University in Boston. The studies proved that the resistance of certain strains of coli bacteria (E. coli) to tetracyclines is due to the expulsion of the drug using energy. Recently, cases have been observed in which "general", wide-ranging flushing mechanisms operate, which are not specific to one drug or a limited group of drugs, but eliminate a broad and diverse range of drugs. The combination of reducing permeability and activating a flushing pump is particularly effective - this way the bacteria can be completely protected from the action of the antibiotic.

The upper picture shows cultures of the bacterium Staphylococcus aureus isolated from patients treated by family doctors. Plates containing antibiotics were placed on the culture medium. It can be seen that most of the bacterial colonies around the plates have disappeared, and the growth medium has become transparent. The reason for this is that these staphylococci are sensitive to most types of antibiotics.
The opposite situation is expressed in the middle picture. Here, "hospital strains" of the same bacteria are grown on the culture medium. Only in one case can full sensitivity to a certain antibiotic be proven, in the same place where the inhibition diameter is large. Staphylococcus strains originating in hospitals have developed resistance to most types of antibiotics.

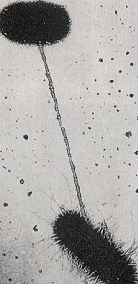
The bottom picture shows the conjugation process between two bacteria. The hereditary material passes from bacterium to bacterium through the thread connecting them.
The active pumps are located in the cell membrane. Thus, in Gram-negative bacteria, the pumps remove the drug, apparently, to the area trapped between the cell membrane and the outer membrane, an area known as periplasm. This kind of washing would not have brought much benefit to the bacteria. And here, it turns out that Gram-negative bacteria have special proteins, which are used, according to the hypothesis, as auxiliary proteins. The assumption is that these proteins cross the periplasm, and through them substances removed by the pump pass out of the outer membrane.
 Kindergarten passes my love
Kindergarten passes my love
How did the bacteria "manage" to overcome drugs, which a lot of work, thought and a huge fortune were invested in their development, leaving the doctors helpless in many cases? How do susceptible bacterial populations acquire resistance to antibiotic drugs? These are, apparently, very effective mechanisms, which managed to surprise even the far-sighted among the researchers.
One of the mechanisms is the most common in evolution - the mechanism of mutations: a change in the composition of the DNA that causes a change in the protein encoded by that DNA segment that changed. Such a protein can be an enzyme, or part of a ribosomal protein structure, for example. If the same protein is the target molecule of the antibiotic drug, a small change in it is enough to make it insensitive to the drug. Thus, following mutations that lead to a change in the components of the ribosomes, bacteria resistant to streptomycin or erythromycin can appear. As a result of mutations causing damage to the bacterial wall proteins, strains resistant to various penicillins can appear. Following a mutation expressed in an RNA-building enzyme (RNA polymerase), strains resistant to antibiotics from the rifamycin group can appear.
A mutation can also expand the range of action of the bacterium's enzyme and allow it to cancel the effectiveness of a new antibiotic, which was developed after the previous one was "defeated" by the bacteria, thus draining labor, investments and hopes. Since the discovery of penicillins, an "arms race" has arisen between the pharmaceutical companies and the bacteria: they have constantly developed new compounds that work against resistant bacteria, and these, for their part, have developed new resistance to the latest "wonder drug" each time. In many cases, it is enough to change one amino acid along the chain that makes up the enzyme, to cause a change in its field of action. A change of one amino acid in a protein results from a change in one triplet of nucleotides along the gene in the bacterium's DNA. In many cases, a change of a single nucleotide changes the meaning of the entire triplet, which is expressed in the amino acid it codes for. A change of a single nucleotide along the DNA is a point mutation. In this context, the story of the penicillin-degrading enzymes that give bacteria resistance to penicillin is interesting: it is possible to follow the genetic "lineages" of these enzymes, which differ from each other by only one amino acid along the long chain, and at the same time trace the expansion of the range of drugs that they break down.
Point mutations can also "convert" enzymes that function within the normal metabolism of the cell - for example in the metabolism of sugars - to those that act on antibiotic drugs and neutralize them. But how can one explain the rapid appearance of enzymes capable of acting on new antibiotic drugs, in bacteria that were initially sensitive to them? It turns out that the emergence of resistance to antibiotics in pathogenic strains often results, not from the creation of a truly new enzyme (a lengthy evolutionary process even when dealing with huge populations and the short generational lives typical of bacteria), but from a process of acquiring genetic resistance that passes from bacterium to bacterium.
How does this happen? In nature, bacteria come into contact with many different antibiotic substances; The very term "antibiotics" indicates the natural origin of these substances - secretions of living beings. Among the bacterial populations that were exposed to natural antibiotic substances for millions of years, enzymes that confer resistance were indeed created. It has long been known that bacteria tend to exchange DNA segments with each other. Today it becomes clear how common this phenomenon is. Like e-mail, bacteria exchange plasmids (independent DNA segments common among bacteria; they include important genes, including genes for resistance). Bacteria also transfer segments of chromosomes. Bacterial viruses also serve as carriers capable of transferring genes from bacterium to bacterium. The various transfer processes are called conjugation (the attachment of bacteria while transferring genetic material), transformation, and transduction (transfer via a bacterial virus).

Colonies of two Staphylococcus species. The largest of which belong to Staphylococcus aureus.
About a decade ago it became clear that conjugation, which is considered a kind of act of mating, surprisingly is not necessarily limited to bacteria that belong to one biological species; It turned out that two bacteria, belonging to different biological species, and even distant from each other, can perform conjugation, for example - when one belongs to an aerial species and its partner is an anaerobic (anaerobic) bacterium. The surprise was even greater, when it became clear that even bacteria on both sides of the "barrier", which for some reason was perceived as impassable: Gram-positive bacteria and Gram-negative bacteria, can perform conjugation and transfer hereditary material. The hereditary material passes, therefore, almost freely between the different bacteria, so much so that instead of thinking of bacteria exchanging genes with each other, it is perhaps possible to think of genes exchanging cytoplasmic hosts for themselves. The soil is a convenient place for bacteria to acquire ready-made genes for themselves, since it infests many species of bacteria. Another good place is our intestines, which are also infested with various bacteria in large numbers.
Bacterial cultures taken from nasal surface.

And a surprising observation: in some cases, exposure of bacteria to an antibiotic increases their tendency to attach to partners and exchange plasmids, which sometimes include genes conferring resistance to that antibiotic itself, and/or to other antibiotics.
And a hypothesis that is perhaps even more surprising: already more than twenty years ago, researchers believed that one of the important potential sources of genes conferring resistance are bacteria that produce antibiotic substances themselves. It turns out that many of the manufacturers of antibiotic substances have enzymes that chemically neutralize their products - this prevents the producing bacteria from "committing suicide" with its own products. Indeed, when they compared the genetic material of antibiotic-producing bacteria and resistant pathogenic strains infesting hospitals, a match was found, indicating that the pathogenic bacteria acquired resistant properties derived from antibiotic-producing bacteria. One of the conclusions that can be drawn from this - a conclusion that actually belongs to the next chapter: it is better to try to produce an artificial antibiotic, which has no equal in nature, because there are no natural producers and there is no source of spreading resistance.
Another conclusion is that if resistance has been discovered in some species of bacteria, it should be expected that within some time it will also spread to other species of bacteria. And in this regard: it turns out that about 20 percent of all enterococci (intestinal bacteria) infesting hospitals are resistant to vancomycin. Hence the great fear - and in fact, the bitter certainty - that eventually this resistance will also reach Staphylococcus aureus - an enemy of the hospitalized, whose strains are currently resistant to all types of antibiotics except vancomycin. And when that happens, medicine will be in great trouble.
The resentment of the experts, the cow's udder and the future of medicine
"If I overdose on my patient's hypertension medication, there is a great fear that I will harm him, but in no way will I harm other patients. On the other hand, if I overdo the dose of an antibiotic, I make it less effective for all the patients who need it." Says Frank Rahm, director of the infection control unit at the University of Minnesota Hospital in the USA, pointing to a paradox and a dilemma. Indeed, the use of antibiotics is a double-edged sword. But the claims and resentment of the experts are not directed only at doctors who prescribe antibiotics too liberally, but also at the "antibiotic gluttons" - basically the majority of the population. A reasonable doctor, who tries to convince his patient that the sore throat originates from a viral infection and there is no point or need for antibiotics, often discovers that the patient will prefer a more "generous" doctor to him in the future...
But the sins of doctors and patients are nullified in sixty (and to be precise - in thirty) in relation to the farmer-veterinarian-cow triangle. Farm animals receive 30 times more antibiotics than humans. In the US, the health authorities allow the existence of 80! Different types of antibiotics in milk (albeit, in low concentrations). They are mainly used to prevent infections in the udders of cows. This means that when drinking a glass of milk, antibiotic substances enter our body. It has been proven that even the low concentrations allowed according to the American standard, increase the rate of appearance of resistant bacteria by six to 72 times.
The experts protest, therefore, against the overuse of antibiotics, both by humans and by the agricultural economy. But it must be remembered that any use, even the most responsible one, eventually triggers the appearance of resistant strains. Therefore, in any case, it is important to promote the evil of resistance. One of the ways is persistence in the "arms race" - to continue developing new drugs to gain time, until resistance to them appears. It turns out that until the mid-XNUMXs, a new antibiotic drug was always on the horizon awaiting approval for use, whereas now the drug companies are no longer making any effort. Even from the government's "higher windows", there are no longer enough funds "flowing" to encourage resistance research and to assist in the development of new antibiotic drugs. It is appropriate to invest a special effort in the development of artificial antibiotics, for which in the first place there is no source in nature to spread resistance - the same bacteria that produce natural antibiotics.
One of the approaches to deal with the problem of the enzymes that confer resistance is to use inhibitors of those enzymes that break down antibiotics. This approach bore fruit in the group of penicillin-degrading enzymes - an inhibitor was found that binds to them and neutralizes their activity. Pills containing penicillin plus the inhibitory substance are marketed in pharmacies - another step in the arms race. But the enemy has already had time to respond: strains of bacteria have already appeared that are resistant not only to the antibiotic drug but also to the added inhibitor...
This is, without a doubt, an exhausting, expensive and dangerous business, and every assistance is needed. It is important to reduce the use of antibiotics to a minimum, and at the same time to be very careful about disinfection and sterilization, in order to reduce the danger of transferring pathogenic bacteria, especially in the hospitals. And perhaps it is also appropriate to reduce the waistline and try to create vaccine components against particularly problematic bacteria.
One should also think about taking sophisticated measures to refresh the effectiveness of the antibiotic drugs. For example, tetracyclines have lost much of their luster due to the emergence of resistance in many of the bacterial species previously known to be sensitive to tetracyclines. In many cases, this resistance is due to an efficient mechanism for washing tetracyclines out of the bacteria. Studies are now being conducted to find a molecule that will block the protein that acts as a pump that removes tetracyclines, thus returning the tetracyclines to their glow.
The most important drug today against the tuberculosis bacterium is isonazid. And here in recent years resistant varieties have appeared. William Jacobs and his colleagues from the Albert Einstein College of Medicine in the US are conducting intensive research to get to the root of the problem. The researchers hope that a thorough understanding of the resistance mechanism will help find an effective way to neutralize it, thus refreshing the effectiveness of the drug for tuberculosis, a serious disease that slowly kills. Will the antibiotic resistance researchers follow them?
It seems that medicine today stands at a crossroads in the glorious history of antibiotic drugs, which in the past have benefited humanity: one path leads to a new momentum, and the other path - to surrender to resistance and degeneration. If so, maybe not "the end of antibiotics", as the Newsweek version, but "antibiotics - where?"
Frame: the wall that encloses the bacterium As in any cell, the cytoplasm of the bacterial cell is also wrapped in a thin cell membrane (membrane), which excels in selective permeability. But unlike the cells of animals, the bacterial cell membrane is protected by the wall that surrounds it from the outside. The cell wall has considerable mechanical strength, which prevents the tearing of the membrane and the explosion of the bacterial cell due to the osmotic penetration of excess water. A protective wall is also known from plant cells, but the chemical composition of the plant cell wall is completely different from the chemical composition of the bacterial cell wall. Damage to the integrity of the wall usually results in water penetration and osmotic explosion of the bacteria. The cell wall, although it excels in considerable mechanical strength, is porous, so it does not constitute a barrier to the passage of small molecules, such as drugs.
The bacteria are sorted according to how they are stained using a staining method known as "Gram staining". Some of the bacteria are dyed purple which cannot be washed off, and are therefore called "gram positive", and some of them lose their color when washed, and are called "gram negative". These differences in coloring point to differences in features, as will be discussed below.
In gram-negative bacteria, the wall is wrapped on the outside in a layer of a sort of cell membrane. This outer membrane - similar to the cell membrane - is selectively permeable, so that many substances, including drugs, have difficulty reaching the Gram-negative bacterium's wall, while their way to the cell wall in a Gram-positive bacterium (which lacks an outer membrane) is open. Inside the outer membrane, which is relatively impermeable, proteins are arranged
Special ones, which create a kind of channels (pores?) (Pore = channel) that facilitate the passage of water-soluble substances. Another difference between gram-positive and gram-negative bacteria is in the thickness of the wall: relatively thick in gram-positive, and relatively little in gram-negative.
Zvi Atzmon, specialized in neurobiology. He teaches life sciences at David Yelin College. He served as the editor of Ladaat and as a senior editor in the Mada system.

One response
The company I own is engaged in marketing and consulting in the field of probiotics and prebiotic food supplements for private companies. We give the professional backing to a single chicken based on the characteristics of its growth. H. As a dietitian of BH, I found a lot of interest in the article, I would be happy to discuss the topic of using probiotic bacteria, in the prevention of diseases in BH. Thanks