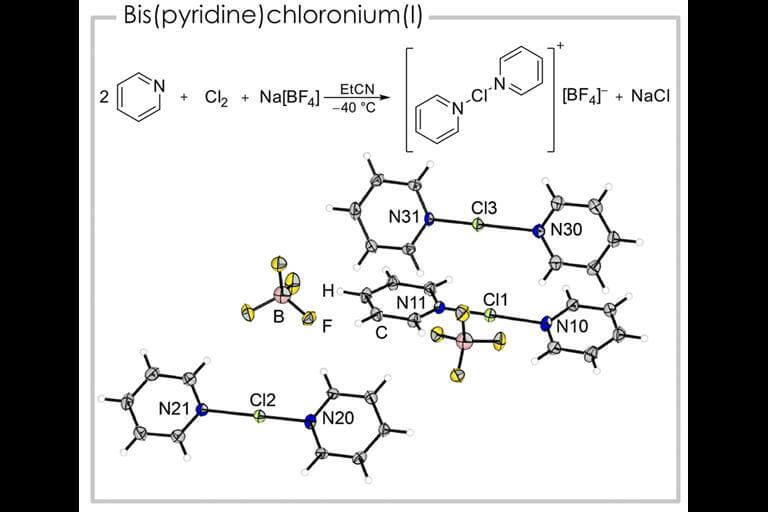
For the first time ever, researchers report the solid structure of pyridine-based chloronium ions. These compounds are known in the literature as intermediates in the oxidation reaction of secondary salts, but until now it has been very difficult to isolate them
[Translation by Dr. Moshe Nachmani]
Halonium ions [Wikipedia] are ions that carry a positive charge on the halogen atom, an element that would normally carry a negative charge due to its high electronegativity. These compounds are known for the first four elements of the halogen group [fluorine, chlorine, bromine and iodine] with the first structural proof for fluoronium reported in 2021. Although the pyridine histories of the halonium ions are all known, and chloronium analogues have been studied using NMR, mass spectroscopy and computer calculations , solid-state structural proof of the chloronium species has not been reported so far.
Sebastian Riedel's research group from the University of Berlin in Germany succeeded in synthesizing the compound [bis(pyridine)Cl][BF4] by reacting pyridine with molecular chlorine and sodium tetrafluoroborate in a propionitrile solvent, with the only byproduct being sodium chloride. The researchers were able to cool the solution to a temperature of minus 80 in order to obtain X-ray images of suitable single crystals. The group was also able to synthesize the compound [mono(pyridine)Cl][AsF6] and obtain its solid structure by adjusting the reaction so that the reactants are pentafluoropyridine and the reactant [Cl2F][AsF6], which is a strong chlorination reagent. A solution of the product in hydrofluoric acid gave rise to single crystals suitable for X-ray analysis. This is the first report of the mono(pyridine)chloronium cation. The news about the research findings
More of the topic in Hayadan:
