The development of nanocomputers that will be used in precision health applications has been the dream of many scientists. Now, for the first time ever, Penn University researchers have succeeded in developing a type of nanocomputer capable of controlling the activity of a specific protein involved in cell movement and cancer development.
[Translation by Dr. Moshe Nachmani]
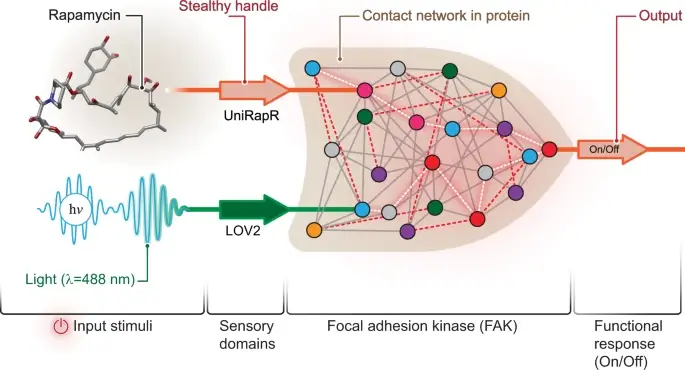
The development of nanocomputers that will be used in precision health applications has been the dream of many scientists. Now, for the first time ever, Penn University researchers have succeeded in developing a type of nanocomputer capable of controlling the activity of a specific protein involved in cell movement and cancer development. The new research paves the way for the development of complex nanocomputers designed for the prevention and treatment of cancer and other types of diseases.
The researchers succeeded in developing a transistor-like 'logic gate', which is a type of computing operation in which several types of input control the type of output. "Our logic gate is just the beginning of the field called 'cellular computing'," explains the lead researcher. "However, our findings are an important milestone in light of the fact that they demonstrate the ability to implement condition-dependent actions in the protein and control its activity," adds and says the lead researcher. "The findings will allow us to gain a deeper understanding of human biology and diseases, and will provide many possibilities for the development of more precise healing treatments."
The state-of-the-art logic gate consists of two sensing complexes adapted to two types of input - light and the drug rapamycin. The researchers focused on the FAK (focal adhesion kinase) protein involved in cell adhesion and movement, which are initial stages in the development of metastatic cancer. "In the first step, we integrated a rapamycin-sensitive compound (uniRapr), which our laboratory had already developed and studied in the past, into the gene encoding the FAK protein," said the lead researcher. "In the next step, we incorporated the light-sensitive LOV2 compound. After we improved the two complexes as much as possible, we combined them together into one common logical gate."
The researchers inserted the modified gene into cancer cells (HeLa) and with the help of a microscope observed the cells in a test tube. They studied the effect of each type of input (light/rapamycin) as well as the combined effects of both types together, on cell behavior. They found not only that they were able to rapidly activate the FAK protein with light/rapamycin, but that this activation also caused the cells to undergo internal changes that increased their adhesion abilities, which ultimately caused them to decrease their ability to move. The findings have long been published in the prestigious scientific journal Nature Communications.. "We have shown, for the first time ever, that we are able to build a functional nanocomputer-type material inside living cells that can control cell behavior," said the lead researcher. We also discovered a number of interesting properties of the FAK protein, such as the changes it undergoes in cells when it is activated." In the next step, the researchers intend to test their innovative system in living cells as well.
More on the subject on the science website:

One response
A similar development was carried out by researchers from Bar Ilan