Are proteins more "exposed" precisely when they are folded?
The life of the protein is dynamic - it is characterized by internal oscillations and a constant exchange of loose hydrogen atoms with water molecules in its environment. The prevailing explanation has always been that these exchanges occur more frequently when the protein is not folded, since the folding prevents water from accessing different areas of the protein. This sounds self-evident, and therefore the rate of hydrogen exchanges was even used as a measure of the degree of folding of the protein, which is an important aspect of the protein's structure and greatly affects its function. BNew research Weizmann Institute of Science scientists have shown, using a new approach based on nuclear magnetic resonance (NMR) combined with nuclear hyperpolarization, that sometimes it is worthwhile to put the obvious to the test.
In order to experimentally examine the exchange of hydrogen atoms between proteins and their aqueous environment, Prof. Lucio Friedman From the Department of Chemical and Biological Physics to the laboratory of Dr Rina Rosenzweig From the Department of Structural Biology. Protein folding is one of the main research topics in Dr. Rosenzweig's laboratory, which specializes in the study of auxiliary proteins that help, among other things, other proteins to fold. The scientists used the "The cold shower” which was developed by Prof. Friedman and his colleagues to enhance the sensitivity of examining biological molecules using NMR, since this method allows studying the interactions between water and protein at an unprecedented level of detail.
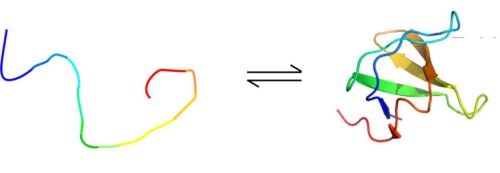
Dr. Or Skelly - who at the time was a research student in Prof. Friedman's laboratory and later a post-doctoral researcher in Dr. Rosenzweig's laboratory - together with Michalo Nowkovitz and Dr. Gregory Olsen, used this method in experiments on four proteins, each of which is characterized by a pattern Different folding. The first protein was well folded, while the second was completely unfolded. The other two proteins continuously switched between a folded and unfolded state, with the scientists controlling the rate of transition using the temperature in the sample. In one of these dynamic proteins, the transitions occurred relatively slowly, about once per second, while in the other protein, the fourth in number, the transitions occurred at a rate of 20 to 30 times per second.
In the first three proteins, the findings were as expected: in NMR, more amplified signals were picked up in the unfolded proteins (or regions) than in the folded proteins (or regions). Thus, for example, in a protein where the transitions between folding and unfolding occurred at a slow rate, the signals increased tenfold in the unfolded state compared to the folded state. But in the fourth protein, where the transitions occurred rapidly, the scientists were in for a surprise: contrary to all expectations, the signal increased much more - three times or more - in the folded parts of the protein compared to the unfolded parts.
"We repeated the experiments over and over again for several months to make sure we weren't wrong," says Prof. Friedman, but the results remain unchanged. The scientists have proposed several theoretical explanations for the surprising findings, and they are planning further experiments to determine which of the explanations is correct. However, even now it seems that the popular information must be updated: it is impossible to automatically assume that the folding of the protein limits its accessibility to the water surrounding it.
