The 2021 Nobel Prize in Chemistry was awarded to two researchers: Benjamin List (Benjamin List) and David WC MacMillan (David WC MacMillan) for "the development of asymmetric organocatalysis". (the news will be updated)
Nobel Prize in Chemistry for 2021
The 2021 Nobel Prize in Chemistry was awarded to two researchers: Benjamin List (Benjamin List) and David WC MacMillan (David WC MacMillan) for "the development of asymmetric organocatalysis".
The two Nobel laureates for this year developed a new and ingenious tool for building molecules: organic catalysis. The field refers to research regarding new drugs and green chemistry. For a long time researchers believed that there were only two types of catalysts: metals and enzymes. Independently of each other, the two laureates for this year developed a third type - asymmetric organic catalysis - based on small organic molecules.
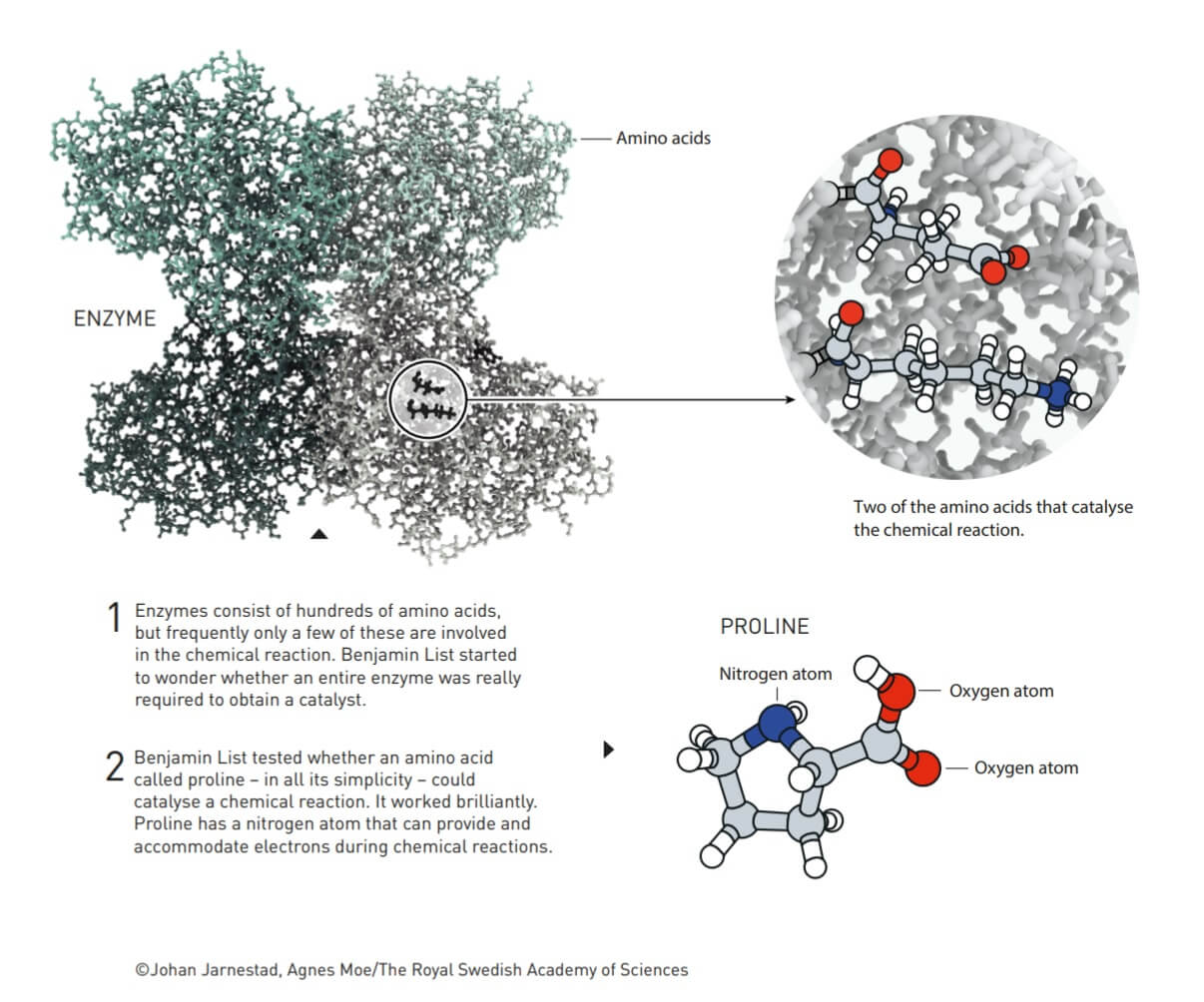
Benjamin List (Benjamin List) wondered if an entire enzyme was really necessary in order to receive catalysis. Oh test whether an amino acid called proline can catalyze a chemical reaction. She did manage to do it.
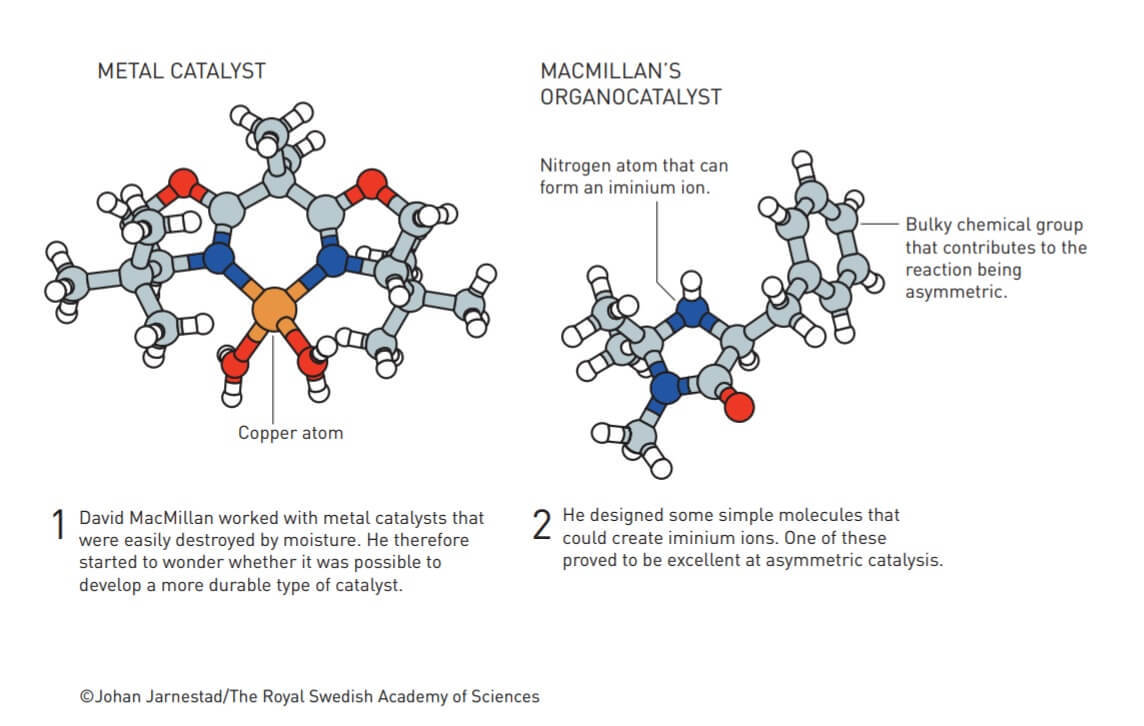
David WC MacMillan worked with metallic catalysts that were easily decomposed by moisture. He wondered if he could develop a more stable type of catalyst using simple organic molecules. One of them proved to be an excellent asymmetric catalyst.
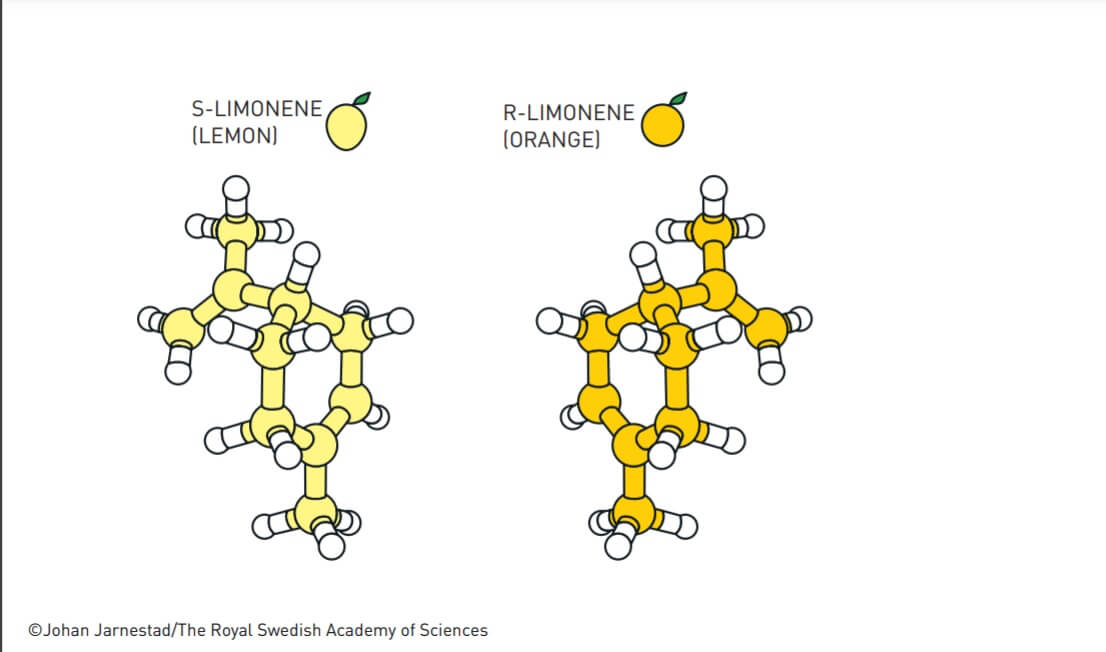
The discovery - asymmetric organocatalysis - for which the Nobel Prize in Chemistry was awarded for 2021, advanced organic synthesis to a completely new level. It not only made chemistry greener, but it made the synthesis of asymmetric molecules much simpler. For example, the two asymmetric molecules of limonene (R enantiomer and S enantiomer) have a slightly different smell: one with a lemon smell and the other with an orange taste. Their synthesis with the help of an asymmetric organometallic catalyst has become simpler today.
The tools developed by the two laureates made a revolution in the construction of molecules
Chemists can create new molecules by joining together small chemical building blocks, but controlling invisible substances so that they bond in the way we need is a challenging and difficult task. Benjamin List (Benjamin List) andDavid McMillan (David WC MacMillan) won the 2021 Nobel Prize in Chemistry for their development of an ingenious new tool for building molecules: organocatalysis. The field refers to research regarding new drugs and green chemistry.
Many industries and fields of research depend on the ability of chemists to build new and functional molecules. These can include everything from materials that capture the sun's rays in solar cells or store energy in batteries, to molecules capable of creating lightweight running shoes or those that inhibit the progression of disease in the human body.
However, if we compare nature's ability to build chemical creations with man's ability, then we were all in the stone age. Evolution has created specially designed tools, enzymes, for the construction of the complex molecular systems that provide life with its structures, colors and functions. At first, when chemists isolated these chemical masterpieces, they were simply in awe of them.
New tools for finer chemistry
Each new tool that chemists added to their toolbox increased the precision of their molecular creations. Slowly but surely, chemists progressed from stirring in stone to something more akin to fine art. This progress has provided great benefit to humanity and some of these tools won their developers the Nobel Prize in Chemistry.
Many molecules exist in two versions, with one being the mirror image of the other. Often these two versions have a completely different effect on our body. For example, one version of the limonene molecule has a lemon scent, while its mirror version has an orange scent.
The discovery for which they won the 2021 Nobel Prize in Chemistry advanced molecular construction to a completely new level. This development not only made chemistry more 'green' (environmentally friendly), but it changed the way to create Asymmetric molecules to a simpler one. During the synthesis of chemical substances, sometimes a situation occurs where two molecules can be formed, which, just like our hands, are a mirror image of each other. Usually, chemists are only interested in one of these two versions, especially when making drugs, but until the time of the discoveries it was difficult to find effective methods for this. The idea developed by Benjamin List and David Macmillan, Asymmetric organocatalysis, is both simple and ingenious.
Catalysts speed up chemical reactions
In the nineteenth century, when chemists began to examine the ways in which different substances react with each other, they made some particularly strange discoveries. For example, if they mixed in silver dust with hydrogen peroxide (H2O2), they noticed that hydrogen peroxide decomposes to form water (H2O) and molecular oxygen (O2). But the money, which initiated the reaction, does not seem to be affected by it at all. Similarly, material taken from germinated seeds was able to break down starch (polysaccharide) into glucose (monosaccharide) molecules.
In 1835, the Swedish chemist Jacob Berzelius began to notice a regular pattern in this field. In one of his articles he wrote: "On a force capable of creating chemical activity". He listed several examples where the mere presence of a substance initiated a chemical reaction, noting that this phenomenon appears to be much more common than previously thought. He believed that such material has catalytic force and called the phenomenon itself catalysis.
Catalysts create plastics, perfume and seasoned food
Ages and ages have passed since the time of Berzelius. Chemists have since discovered a wide range of catalysts capable of breaking molecules apart or joining them together. Thanks to them we are now able to create the thousands of different materials that we use in our daily lives, such as medicines, plastics, perfumes and seasonings. It is estimated that about thirty-five percent of the global GDP in one way or another is related to chemical catalysts.
Basically, all catalysts discovered before the year 2000 belong to one of two groups: they were metals or enzymes. Metals are usually excellent catalysts because they have the unique ability to temporarily accumulate electrons or transfer them to other molecules during a chemical reaction. This phenomenon helps to weaken the chemical bonds between the atoms in the molecule, so that bonds that were otherwise tight can now be loosened, while new bonds are formed in their place.
However, one of the common problems with some of the metal catalysts is that they are very sensitive to oxygen and water, so their use requires an environment devoid of oxygen and moisture. In addition, many metallic catalysts are based on heavy metals which are dangerous substances for humans and the environment.
The catalysts of life work with amazing precision
The second type of catalyst consists of proteins known as enzymes. All living things have thousands of different enzymes that drive the chemical reactions required for life. Many enzymes specialize in asymmetric catalysis and, in principle, always create only one of the two possible versions of the mirror images. In addition, they work simultaneously - when one enzyme ends its activity in a certain reaction, another enzyme starts its own activity. In this way, they are able to assist in the synthesis of complex molecules with incredible precision, molecules such as cholesterol, chlorophyll or the toxin known as strychnine, which is one of the most complex molecules known to the world of science.
Since enzymes are such effective catalysts, researchers in the nineties of the last century tried to develop new enzymes in order to speed up the chemical reactions important to humanity. One of the research groups working in this field was a group led by Carlos F. Barbas III at the Scripps Research Institute in Southern California. Benjamin List was at the time a PhD student in Barbas's research group when his genius idea, which led to one of the discoveries behind the Nobel Prize in Chemistry this year, was born there.
Benjamin List thinks outside the box
Benjamin List worked with catalytic antibodies. Normally, antibodies bind to foreign viruses or bacteria in our bodies, but the Scripps researchers have re-engineered them so that they can drive chemical reactions instead.
During his work with catalytic antibodies, Benjamin List began to wonder how enzymes really work. These are usually large molecules consisting of hundreds of amino acids. In addition to these amino acids, many enzymes also have metals that help drive chemical processes. However - and this is the crux of the matter - many enzymes catalyze chemical reactions without the help of metals. Instead, the reactions are driven by one or more amino acids within the enzyme. Benjamin List's unique question was: Do amino acids have to be part of an enzyme in order to catalyze a chemical reaction? Or can just one amino acid, or other similar simple molecules, do the same job?
A breakthrough result
Liszt knew from research conducted back in the seventies that an amino acid called proline was used as a catalyst - but this finding was over 25 years ago. Surely, if proline was indeed used as an effective catalyst, someone would have gone on to investigate it by now, wouldn't they?
For a while Benjamin Liszt thought more or less; He estimated that the reason why no one had continued to investigate this phenomenon was that the catalysis did not work properly. Without any real expectation, he began to test whether proline could catalyze an aldol reaction, during which carbon atoms from two different molecules bond together. It was a simple attempt that surprisingly worked right away.
- Enzymes are made up of hundreds of amino acids, but usually only a small part of them are involved in the chemical reaction. Benjamin List began to wonder whether an enzyme was indeed necessary in its entirety in order to achieve the catalytic activity.
- Benjamin List wondered whether an amino acid called proline - for all its simplicity - could catalyze a chemical reaction. It worked wonderfully. Proline has a nitrogen atom that is able to provide and deal with electrons during chemical reactions.
As part of his experiments, Benjamin List not only demonstrated that proline is an effective catalyst, but that this amino acid can initiate asymmetric catalysis. Of the two possible mirror images, one of them was created in greater quantity than the other. Unlike researchers who had previously examined proline as a catalyst, Benjamin List understood the tremendous capacity that this acid could have. Compared to metals and enzymes, proline is a dream tool for chemists. It is a very simple, cheap and environmentally friendly material. When he published his discovery in 2000, List described asymmetric catalysis with the help of organic molecules as a new idea that holds many opportunities: "The design and screening of these catalysts is one of our future goals." However, he was not alone in this field. In his laboratory further north in California, David McMillan also worked for this purpose.
David McMillan Neglects to comfort the sensitive metals
Two years earlier, David McMillan had moved from Harvard University to Berkeley. At Howard he worked on improving asymmetric catalysis with the help of metals. It was an area that attracted a lot of attention from researchers, but David McMillan noticed that the catalysts that were indeed developed were hardly ever used in industry. He concluded that the sensitive metals were simply too complex and expensive to use. Achieving oxygen-free and moisture-free conditions required for several metal catalysts is quite simple in the laboratory, but carrying out industrial processes under such conditions is quite complex. His conclusion was that if the chemical tools he developed were to be effective, he had to rethink. So, that when he moved to Berkeley he left the metals behind.
Then he developed a simpler type of catalyst
Instead, David McMillan began designing simple organic molecules which - just like metals - could temporarily donate or accept electrons. At this stage, he is required to define what organic molecules are - in short, these are the molecules that create all living things. They include a fixed skeleton of carbon atoms to which are attached active chemical groups that usually contain oxygen, nitrogen, sulfur or phosphorus atoms.
Organic molecules, therefore, consist of simple and common elements, however, depending on how they are connected, they can have complex properties. David McMillan's knowledge of chemistry led him to the conclusion that in order for an organic molecule to be able to catalyze a required reaction, it must be able to form an iminium ion. This ion includes within it a nitrogen atom that has an inherent affinity for electrons.
He selected several organic molecules with the required properties, then he tested their ability to catalyze the Diels-Alder reaction that chemists use to form rings of carbon atoms. As he hoped and believed, the reactions worked amazingly. Some of the organic molecules also worked well as asymmetric catalysts. Of the two mirror images, one of them made up more than ninety percent of the final product.
David McMillan drowns the term organic catalysis (organocatalysis)
When David McMillan was ready to publish his findings, he realized that the type of catalysis he had just discovered needed a name. The fact was that researchers did succeed in the past in catalyzing chemical reactions with the help of small organic molecules, but these were isolated examples, and none of the researchers understood that this was a general method. David McMillan wanted to find a term that could describe the method in such a way that other researchers would understand that there were still many catalysts to be found. His choice was organic catalysis. In January 2000, just before the publication of Benjamin List's discovery, David McMillan submitted his article for publication in a scientific journal. The introduction was: "Hereby, we present a new strategy for organic catalysis that we expect to be suitable for a spectrum of asymmetric conversions".
The use of organic catalysis flourished and flourished
Unrelated to each other, Benjamin List and David Macmillan discovered an entirely new idea for catalysis. Since the year 2000, the developments in this field are really similar to the developments that were in the gold rush era, when these two researchers were at the head of the arrow. They designed a wealth of cheap and stable organic catalysts that could be used to catalyze a huge variety of chemical reactions.
Not only do organic catalysts often consist of simple molecules, in some cases - just like nature's enzymes - they can act just like on an industrial conveyor belt. In the past, as part of chemical production processes, it was necessary to isolate and purify each of the intermediate products in order to avoid receiving many by-products. These restrictions caused a reduction in the utilization of these chemical processes.
Organic catalysts are much more forgiving, since several reaction steps can be carried out with them in sequence. It is a cascade reaction that significantly reduces the amount of waste in chemical production.
Today's synthesis of styrchnine is 7000 times more efficient than before
One example of how organic catalysis led to more efficient molecular construction is the synthesis of the strychnine molecule, which occurs in nature and has an extremely complex structure. For chemists this molecule is similar to a Hungarian cube: a challenge you want to solve in the fewest number of steps. When strychnine was first synthesized in 1952, it was produced by 29 different chemical reactions, and only 0.0009 percent of the starting material eventually became the desired product. The rest was waste. In 2011, researchers were able to use organic catalysis and a cascade reaction to produce strychnine in just eleven steps, with the production process being 7000 times more efficient.
Organic catalysis is particularly important in the field of pharmaceutical production
Organic catalysis has had a significant impact on drug research, where asymmetric catalysis is often required. Until the moment when chemists could carry out asymmetric catalysis, many of the drugs contained both forms of the structure of the active substance; One of them was active, while the other might prove to be responsible for side effects. A catastrophic example of such a case was the thalidomide scandal in the sixties, in which one of the forms of the molecule caused serious deformities in thousands of developing embryos.
With the help of organic catalysis, researchers can now produce large quantities of asymmetric molecules quite simply. For example, they are able to synthesize healing substances that are otherwise only obtainable by isolating them in only minute quantities from rare plants or marine organisms.
In pharmaceutical companies, the method is also used to increase the production of existing drugs. Examples of this include the drugs paroxetine, which is used to treat anxiety and depression, and the antiviral drug oseltamivir, which is used to treat respiratory viral infections.
Simple ideas are the hardest to imagine
One could list thousands of examples of how organic catalysis is used - but why didn't anyone discover this simple, green and cheap idea of asymmetric catalysis earlier? This question has many answers. One is that simple ideas are often the hardest to imagine. Our perspective is obscured by significant preconceptions about how the world should work, such as the idea that only metals or enzymes can catalyze chemical reactions. Benjamin List and David McMillan were able to see beyond these preconceived notions while finding an ingenious solution to a problem that chemists had struggled with for decades. Organic catalysis, as a result, provides - right now - the greatest benefit to mankind.
More of the topic in Hayadan:
