The discovery of a mysterious helical lock may shed light on the development of degenerative brain diseases
Proteins are the workhorses of the cell that carry out the vast majority of the tasks necessary to keep us healthy and whole. However, damaged proteins can form harmful aggregates in the brain that are involved in Alzheimer's, Parkinson's and other degenerative brain diseases. Until recently, the popular belief was that once these aggregates, known as amyloid deposits, are formed, there is no turning back. But it turns out that cooperation between two families of accompanying proteins ("chaferons") makes it possible to break down the amyloids and keep our body cells in a normal state.
In two recently published articles, the laboratory of Dr Rina Rosenzweig from the Department of Structural Biology, together with researchers from the University of Heidelberg and the German Cancer Research Center (DKFZ), the mechanism by which the two protein families break down the amyloid deposits. This action fits well into the general task of the champrons in the cell: to maintain the integrity of proteins throughout their functional life.
In the first article, Dr. Ann Vantinck from the group of Prof. Brand Bokaw at the University of Heidelberg discovered that in order to break down the amyloid deposits, many Hsp70-type spheromons must gather together at the same point, stick to individual proteins within the amyloid cluster and pull them out until they are freed from the tangle. In the second article, Dr. Ofra Faust and Dr. Mital Aviv-Abraham from Dr. Rosenzweig's group discovered a unique control mechanism that allows the second family of chaperones, Hsp40, to initially produce the same Hsp70 cluster that is necessary to break down amyloids.
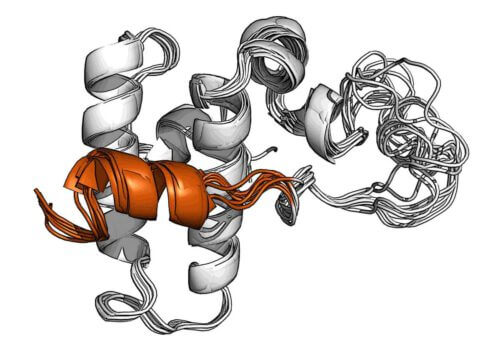
Deciphering the DnaJB1 protein structure
Through advanced nuclear magnetic resonance experiments at the Klor Institute for Imaging and high-intensity magnetic resonance spectroscopy, Dr. Rosenzweig's group, in collaboration with Dr. Nir London from the Department of Organic Chemistry, succeeded in deciphering the structure of a certain protein from the Hsp40 family known as DnaJB1. Decoding the structure showed that DnaJB1 contains a unique helix that temporarily blocks the binding site for the Hsp70 molecule. This helix is part of a two-step control mechanism that was not recognized until now, and it is this that allows this hexagram, if only, to recruit molecules from the Hsp70 family in the manner necessary to break down the amyloids.
In order to begin the work of degradation, Hsp70 must first be activated by DnaJB1, which is already bound to amyloid, but the binding site is blocked by the helical lock. Thus, Hsp70 first binds to heparin at another site, through its unregulated region, a kind of molecular "tail". This action releases the helical lock and allows Hsp70 to bind to DnaJB1 at the primary binding site, thus becoming active and starting the job of breaking down the amyloid. After some time the helical lock closes, Hsp70 is released and DnaJB1, which is still attached to the amyloid, is freed to capture new Hsp70 proteins and activate them in the same area. This repetitive action eventually produces the critical mass of Hsp70 proteins needed to break down the amyloid deposit.
This new understanding of the molecular mechanisms that act to break down amyloids raises the question of whether the development of neurodegenerative diseases is accompanied by a disturbance in this mechanism - a question whose answer could pave a new way to combat diseases such as Alzheimer's and Parkinson's. Moreover, the research findings may make it possible to understand other vital processes in the cell, as the scientists believe that similar helical locks are used in other spherons of the Hsp40 family.
