Understanding the mechanism is expected to help in the development of new anti-obesity drugs
Eating non-stop and staying hungry all the time - for most of us this sounds like a nightmare, but for people with genetic defects in the appetite control mechanisms in the brain, it is a grim and everyday reality that often leads to extreme obesity. in research published this week in the scientific journal Science, scientists from the Weizmann Institute of Science, in collaboration with scientists from Queen Mary University of London and the Hebrew University of Jerusalem, deciphered the mechanism of action of the main switch of the feeling of hunger and satiety in the brain: the melanocortin 4 receptor or MC4 for short. These findings shed new light on the control mechanisms of the hunger and satiety processes in our body and are expected to help in the development of new anti-obesity drugs.
The MC4 receptor is a small protein expressed in nerve cells in the area of the brain called the hypothalamus, as part of a cluster of cells (one of which is called the paraventricular nucleus) that calculates the energy balance in the body by sensing and processing various metabolic signals. When MC4 is in an active state - and this is its natural state - it sends commands that make us feel full; That is, as far as the brain is concerned, the human body's default is satiety. When our energy levels drop, the cluster of cells in the hypothalamus secretes a hormone that turns off the MC4 receptor and makes us feel hungry. After the meal, another hormone is secreted that binds to the same binding site on the surface of the receptor, removes the hunger hormone from it and returns the receptor to its active state, i.e. to the feeling of satiety. Mutations that disable MC4 cause people to feel constantly hungry.
The present study began following the plight of an Israeli family in which more than seven family members suffer from constant hunger and extreme obesity; In fact, for most of them, the body mass index (BMI) stands at a value higher than 70 - that is, about three times the norm. Hadar Israeli, a medical student researching the mechanisms of obesity as part of her doctoral thesis under the guidance of Dr. Dani Ben-Zvi at the Hebrew University of Jerusalem, became acquainted with the family during her studies. Israeli, who was amazed that the family's distress stems from only one mutation in the MC4 receptor, turned to Dr. Moran Shalu Ben-Ami from the Department of Structural and Chemical Biology at the Weizmann Institute, specializing in XNUMXD imaging of receptors in the central nervous system.
Following her application, Dr. Shalu Ben-Ami offered the Israeli to join her laboratory as a guest scientist and investigate, using state-of-the-art electron microscopy technologies, how the receptor works and why exactly this mutation leads to such serious consequences in patients. Israeli and Dr. Oksana Dagitarik, a postdoctoral researcher in the laboratory, isolated large amounts of MC4 receptors from cell membranes, and deciphered the three-dimensional structure of the receptor using a cryogenic electron microscope. The research was conducted in collaboration with the group of Prof. Masha Niv from the Hebrew University and Dr. Peter McCormick from Queen Mary University in London.
The current study began following the plight of an Israeli family in which more than seven family members suffer from constant hunger and extreme obesity
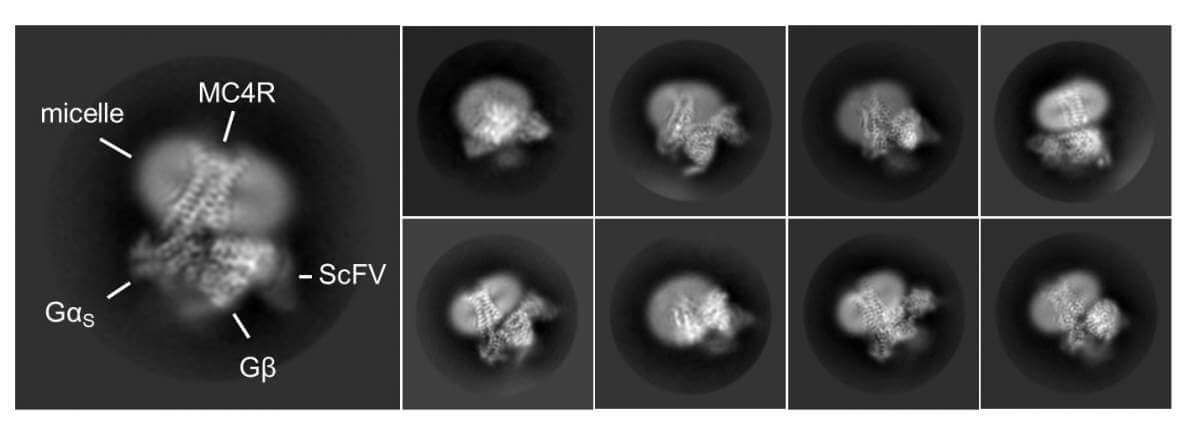
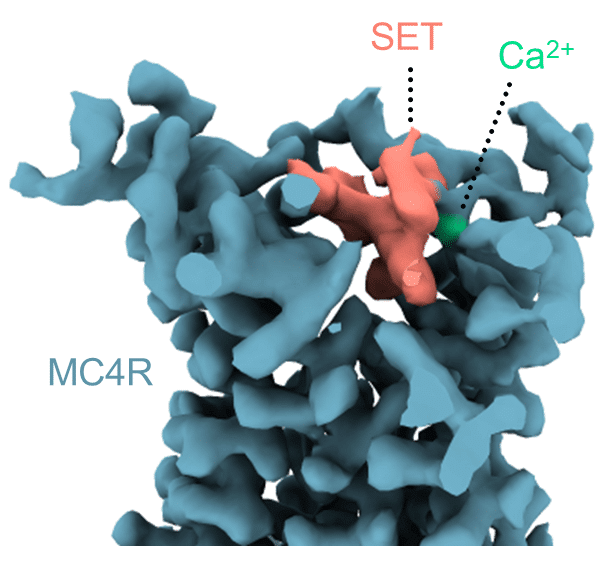
The fact that the MC4 receptor practically controls the body's feelings of hunger and satiety makes it a central target for the development of anti-obesity drugs. As part of the study, the research team also revealed the mode of action of a new drug called Stemlanotide (Imcivree) that was recently approved by the American Food and Drug Administration (FDA) to treat cases of obesity resulting from certain genetic defects. The researchers revealed that the drug binds to the receptor and directly activates the satiety switch in the brain - in fact, it does so in an even more powerful way than the natural satiety hormone. In addition, the identity of a surprising assistant that helps the drug was revealed: a calcium ion that strengthens the binding of the drug to the receptor. In biochemical and computational experiments, the scientists found that calcium similarly helps the natural satiety hormone to activate MC4, while at the same time disrupting the action of the hunger hormone.
"It was an extremely surprising finding," says Dr. Shalu-Ben Ami. "It seems that with the help of calcium, the satiety signal can successfully compete with the hunger signal, thus helping the brain to return to a feeling of satiety after the meal."
In addition, the scientists identified structural features that distinguish MC4 from similar receptors of the same family. This identification will make it possible to develop more targeted drugs that will bind only to MC4 without activating other receptors and causing side effects. "Our findings will help promote the development of improved and safer anti-obesity drugs," says Dr. Shalu-Ben Ami.
Dr. Fabrizio Piero from the Hebrew University of Jerusalem participated in the study; Vidiche Chunilal, Amandeep Rauk Gill, Nicholas Roth, Dr. Joaquin Botha and Dr. Lee Chan from Queen Mary University of London; Dr. Vadival Prabhar from the Department of Structural and Chemical Biology at the Weizmann Institute, and Dr. Yoav Peleg from the Department of Life Science Research Infrastructures at the Institute.
More of the topic in Hayadan:
- Scientists have identified a hormone that causes a feeling of satiety
- First findings of a unique study: Are the gyms closed? The Israelis continue to gain weight
- Is there a connection between obesity and choosing a partner in Israel? Israeli research reveals fascinating results
- The gut feeling of the ant
