Why can chronic intestinal diseases lead to colon cancer?
Chronic inflammation is a favorable substrate for the development of some of the deadliest types of cancer. Colon cancer, for example, is more common in people with inflammatory bowel diseases, such as Crohn's and colitis. Although this fact is known to doctors, many times these people will only be diagnosed with cancer in an advanced stage of the disease, as the initial warning signs may be mistakenly attributed to the inflammatory condition. BNew research Weizmann Institute of Science scientists have revealed an important molecular link that links chronic intestinal inflammation to cancer. These findings may help in the development of treatment to prevent cancer in people with inflammatory bowel diseases.
Dr Ruth Shertz-Shuval from the Department of Biomolecular Sciences hypothesized that the pathway leading from chronic intestinal disease to cancer passes through the intestinal cells' response to inflammation - and in particular the action of a protein called HSF1. In a previous work, Dr. Shertz-Shouvel discovered that this protein, which is activated in stressful situations, causes fibroblasts - supporting cells that build the intercellular tissue - to mobilize for the cancer cells in their environment. In the new study, she and her group set out to check if this process begins even before the appearance of cancer, as part of the inflammatory state.
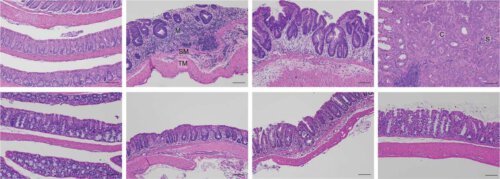
To this end, Osherat Levy-Glibov, a research student in Dr. Shertz-Shovel's group, led a series of experiments in which she monitored changes in mice with chronic intestinal inflammations that later developed into cancer. The research team examined the intestinal tissue of the mice at different stages of the inflammatory disease using advanced two-photon microscopy, and examined the composition of the proteins in this tissue using mass spectroscopy and other methods. The scientists discovered that already in the initial stages of inflammation, the intercellular tissue - that supporting network made of collagen and other large molecules - underwent abnormal changes that contributed to the development of cancer. To test whether HSF1 is the cause of these changes, the protein was silenced through genetic engineering - and it was discovered that the fibroblasts built a normal intercellular tissue, and the engineered mice did not develop chronic inflammation or bowel cancer.
Later, the scientists were able to show that the findings are also valid in humans. In collaboration with the Sheba Medical Center (Tel Hashomer) and the Memorial Sloan Kettering Cancer Center in New York, the scientists examined tissue samples taken from colon cancer patients with a history of inflammatory bowel disease. The scientists discovered that HSF1 was activated in the tissues of these patients, and that the intercellular texture and protein composition underwent abnormal changes similar to those observed in mice.
To test the possibility of a future treatment, the scientists exposed fibroblasts isolated from the inflamed tissue to a synthetic molecule that blocks the activity of HSF1. Following this exposure, the supporting cells stopped producing intercellular tissue - that is, the molecule put an end to the cancer-promoting activity. These findings may in the future lead to a drug that will prevent the development of colon cancer in people with inflammatory bowel diseases by selectively blocking the effect of HSF1. In addition, the findings may make it possible to identify which of the patients with inflammatory bowel diseases are at high risk of developing cancer, and to give them preventive treatment ahead of time.
Hagar Levon, Dr. Rina Wasserman-Dozortez, Dr. Mirav Pevsner-Fisher, Shimarit Meir, Yaniv Stein, Gil Friedman and Dr. Rinat Nebo from the Department of Biomolecular Sciences at the Weizmann Institute of Science participated in the study; Dr. Esther Warshoff and Prof. Eric Sahai from the Francis Crick Institute, London; Dr. Lauren A. Brown, Dr. Wenhan Zhang and Prof. John Furco of Boston University; Ofra Golani from the Department of Life Science Research Infrastructures at the Weizmann Institute of Science; Dr. Lior Katz, Dr. Ido Leish and Dr. Dror Shuval from Sheba Medical Center; and Dr. Rona Yeager and Dr. David Klasen from the Memorial Sloan Kettering Cancer Center in New York.
More of the topic in Hayadan:
