In a series of experiments, Weizmann Institute of Science scientists solved the puzzle of the relationship between electric current and the freezing temperature of supercooled water. Beyond the scientific interest, their findings may lead to the advancement of applications in diverse fields where control of ice formation is required

In science lessons at school we learned that water freezes at 0 degrees Celsius, but the reality, as usual, is more complex. Although ice turns into water at 0 degrees, water in a state of "supercooling" can remain liquid even at 40 degrees below zero. Another fact that complicates matters: "supercooled" water freezes immediately when exposed to an electric current, as the physicist Louis Dufour discovered in 1861.
This phenomenon, called "electrofreezing", remained a mystery for more than 150 years. Recently, bA series of experiments, Weizmann Institute of Science scientists solved the puzzle of the connection between electric current and the freezing temperature of supercooled water. Beyond the scientific interest, their findings may lead to the advancement of applications in diverse fields where control of ice formation is required - from freezing food and freezing organs for transplantation to preventing the formation of ice on airplane wings and seeding clouds.
Ice icicles usually form around tiny crystals called "freeze nuclei". In previous studies, the institute's scientists showed that polar crystals, which are pyroelectric - that is, those whose surface, at both poles, are electrically charged when they are heated or cooled - produce ice around them at a higher temperature than non-polar crystals. About two decades later, the institute's scientists showed that negative or positive electric charges affect the freezing temperature differently. However, the link between electricity and freezing remains a mystery.
In the new study, a group of scientists led by Prof. Igor Lubomirsky, Prof. Meir Lahav and Dr. David Ahara from the Department of Materials and Surfaces if "electrical freezing" occurs due to the electric charge or due to the geometric properties of the charged crystals. The experiments, led by Dr. Sophia Kurland, then a doctoral research student, and master's student Lea Shavit, focused on pyroelectric crystals and were carried out in a unique device that allowed scientists to change the charge on the surface of the crystals without changing their other properties.
When the scientists cooled the crystals, they discovered a surprising relationship between the freezing temperature, the degree of polarization and the acidity level of the water droplets that condensed on the surface of the crystal: while in the polar crystals, when the acidity level was high, the freezing temperature was 3 to 5 degrees Celsius higher , in the non-polar crystals, the freezing temperature was the same regardless of the acidity level. What caused this difference between the polar and non-polar crystals?
The scientists discovered that during cooling, a thin and continuous layer of water forms on the surface of the hydrophilic ("water-loving") surfaces of the pyroelectric crystals, and within this layer there is an electric current of ions created as a result of the absorption of carbon dioxide (CO2) from the air which produces ions of hydrogen carbonate (bicarbonate), a derivative of carbonic acid, in the water. A large amount of these ions is what increases the acidity of the water. In other words, it turned out that the connection between the freezing and the electric charge is chemical: the freezing at a higher temperature was due to the amount of bicarbonate ions. When the scientists repeated the experiments with hydrophobic ("water-hating") crystals, a continuous layer of water did not form on their surface and the freezing temperature remained the same regardless of the acidity level.

These findings are surprising in several respects: first, according to the chemistry textbooks, mixing water with foreign ions should lower their freezing temperature, but the absorption of CO2 caused the opposite phenomenon. Furthermore, freezing requires crystals with a geometrical structure similar to that of ice, so how, then, was the formation of ice affected by bicarbonate molecules?
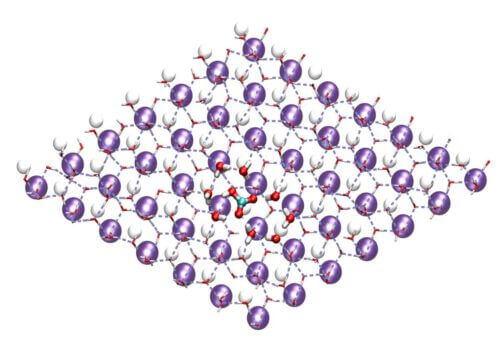
In order to answer these questions, the scientists of the institute collaborated with Prof. Daniel Harris and Dr. Christoph Aluliou from the Hebrew University of Jerusalem. They made calculations accompanied by computer simulations, and found that in the thin layer of water near the charged surface of the crystal, the bicarbonate ions, together with the water molecules, organize themselves in stable hexagonal structures similar to the structure of the hexagons of the ice crystals, and therefore serve as freezing nuclei for the formation of ice.
Following these experiments, the scientists discovered that there are two types of ions: those similar to bicarbonate, with a triangular structure (symmetrical in three directions in a certain plane), which raise the freezing temperature of water, and ions that do not have such a structure, which lower the freezing temperature.
The findings were published in a special issue of the scientific journal applied Chemistry, imply that the increase in CO levels2 The atmosphere may affect the climate in surprising ways. Rain falls when ice crystals form in the clouds around dust particles that serve as freezing nuclei. Most of these particles have an electric charge, and because they may be in mutual relations with CO molecules2, an increase in the concentrations of this greenhouse gas can lead to increased rainfall in the world - a phenomenon that may require adjustments in climate models.
The research findings may also be relevant in a variety of applications that include water freezing. For example, they are already helping to understand how the crystals of silver iodide, currently used in cloud seeding, lead to increased rain: not only do they have a geometric structure similar to ice, but also, being pyroelectric, their surface attracts CO2 Thus raising the formation temperature of the ice in the clouds. Moreover, the research findings may help find pyroelectric materials that are more environmentally friendly than silver iodide.
Dr. Isabella Weisbuch and Shiri Dishon Ben Ami from the Department of Materials and Surfaces of the Institute participated in the study.
