The surprising discovery of scientists from the Weizmann Institute of Science and Yale University opens up new possibilities for the treatment of diabetes
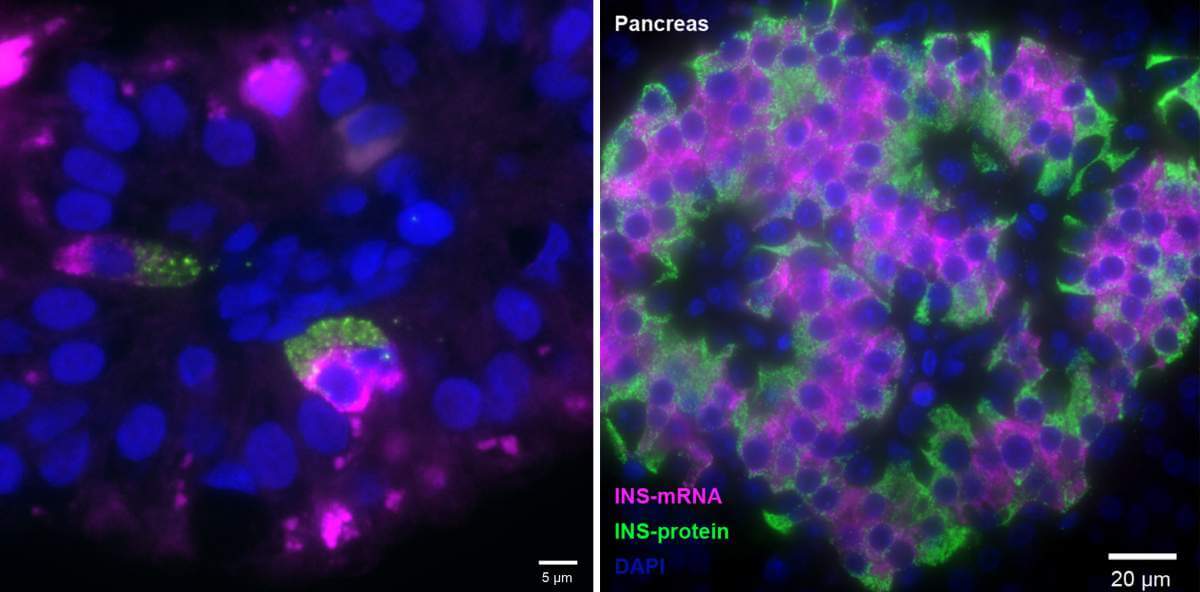
The exclusive "franchise" for producing insulin in the human body is reserved for beta cells in the pancreas - at least that's what is commonly thought. in research published today in the scientific journal Nature Medicine, scientists from the Weizmann Institute of Science and Yale University in the United States discovered cells that produce the essential hormone for regulating blood sugar levels in an unexpected place: the fetal intestine. The discovery opens up new directions for the development of treatments for diabetes, which affects hundreds of millions of patients worldwide.
In the developing embryo there are many cells that have not yet decided what they will do when they grow up. This is especially true for the cells in the intestines: this digestive organ develops in the second trimester of pregnancy, but as long as the fetus is fed through the umbilical cord, the fetal intestine is disabled from its traditional functions, and its cells can occupy themselves with extraordinary tasks (while waiting for food to arrive).
To locate these unusual activities in the fetal intestine, Prof. His Itzkowitz From the Department of Molecular Biology of the Cell at the Institute of Prof. Liza Konnikova from the Yale School of Medicine. A joint research team for both groups led by Adi Aguzi, a PhD student in Prof. Itzkovitz's group, first mapped the gene expression profile in the intestinal cells of embryos using advanced technology for sequencing the genetic material at the single cell level in thousands of cells simultaneously. The researchers compared the gene expression map obtained from 36 fetal intestinal cells to a corresponding map in newborn babies.
""It is not clear how insulin production in the intestine stops after birth, but it is possible to awaken this dormant program in diabetics"
The comparative study revealed a surprising discovery: cells outside the pancreas expressed the gene for insulin production in some of the embryos. These were cells of the K/L type - cells that secrete hormones in the small intestine. These cells in the fetal small intestine, henceforth called FIKL, expressed not only the gene that produces insulin but an entire molecular program designed to support the activity of this hormone, including genes that function as sugar sensors or those responsible for secreting the hormone from the cell. In addition, similar to beta cells, FIKL cells produced insulin in extremely large quantities and it was discovered that the organization of the molecules in both types of cells is similar: the messenger RNA molecules, which hold the recipe for insulin production, were closer to the inside of the cell - while the hormone itself accumulated on the outside of the cell when ready and ready for deployment.
"The similarity between beta cells and FIKL cells is not entirely surprising, since the pancreas and the small intestine are formed from the same fetal tissue around the fifth week of pregnancy," says Prof. Itzkovitz. However, it is not yet clear what the role of insulin-producing cells in the fetal gut is. It is possible that the genetic program for the production of the hormone was activated in the fetal cells in response to the mother's gestational diabetes or that the production of insulin was intended for "local" purposes: supporting the rapid development of the intestines. It should be noted in this context that the cells tested in the study were taken from a fetal tissue bank - and the researchers have no information about the health status of the mothers and their sugar levels during pregnancy.
"It is not clear how insulin production in the intestine stops after birth, but it is possible to wake up this dormant program in diabetics," says Prof. Itzkovitz. "If this becomes possible, cells from the intestinal mucosa may be a wonderful source of insulin production, as they are constantly renewed."
In diabetes, beta cells in the pancreas are destroyed by the immune system ("juvenile diabetes" or type 1 diabetes) or alternatively collapse under the body's sugar load (type 2 diabetes). Researchers trying to cure diabetes through cell transplants have so far mainly used stem cells or genetically engineered cells that have been made to produce insulin. "The advantage of the cells we discovered in embryos is that they naturally contain the action plan for producing insulin," says Prof. Konnikova. "If we manage to identify the molecular signals that turn this program on or off, we may one day be able to activate it when needed."
Participants in the study were Dana Lieviciocha-Loha and Blake McCourt of the Yale School of Medicine; Dr. Keren Behar Halpern and Dr. Lydia Parak from the department of molecular cell biology of the institute; and Xiaojing An, Fujing Wong and Dr. Kong Chen from the University of Pittsburgh.
