The researchers from Harvard and MIT who developed an innovative device capable of detecting the corona virus in a saliva sample in about an hour claim that the reliability of the diagnosis is similar to that of PCR tests
[Translation by Dr. Moshe Nachmani]
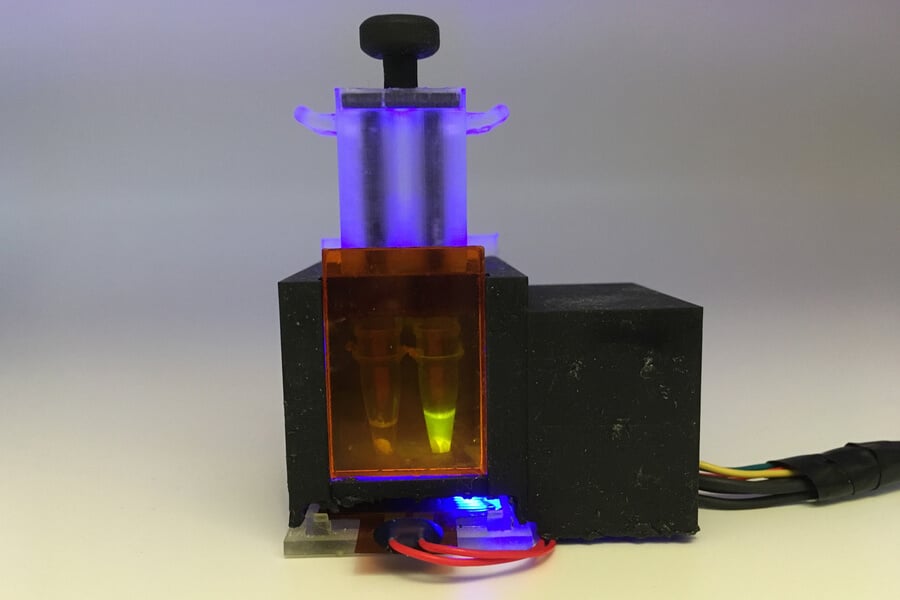
Engineers from Harvard University and the Massachusetts Institute of Technology (MIT) have succeeded in developing a small desktop device capable of detecting the corona virus from a saliva sample in about an hour. In a new study, the developers showed that the level of diagnostic reliability of their method is just as accurate as the level of reliability of PCR tests. The device is also able to detect specific viral mutations associated with the variants of the corona virus that are spreading today. The result is obtained in about an hour, a fact that makes the method simpler in terms of tracking different versions of the virus, especially in areas where there is no access to facilities or equipment for genetic sequencing. "We were able to demonstrate that our method can be tuned so that it can detect new versions of the virus that pop up here and there, and that it can be done quickly," said the lead researcher. "As part of this study, we focused on those variants that spread in the UK, South Africa and Brazil, but it is certainly possible to adapt our diagnostic method in order to deal with the delta version and variants that will emerge in the future." The cost of the innovative diagnostic method, based on the CRISPR technology (the term in Wikipedia), reaches only fifteen dollars, and this cost is possible at least if the devices are produced on a large scale, the researchers claim. The new study has long been published in the scientific journal Science Advances.
The new diagnostic method is based on a device called SHERLOCK, based on CRISPR, about which the same researchers published back in 2017. The components of the system include a guided RNA strand that enables the identification of specific RNA sequences, and Cas enzymes that cut these sequences while emitting fluorescent light. All these molecular components can be freeze-dried to store them for a long time and then be reactivated when necessary with the help of exposure to water. Last year this research group began to explore the possibility of adapting the technology to detect the corona virus as well, hoping to succeed in designing a diagnostic device that could provide rapid results without the need for any previous training or experience.
The aim is to diagnose the virus in saliva samples. In order to achieve this goal, the researchers had to implement an essential preliminary step that inactivates enzymes called 'salivary nucleases', whose normal function is to break down nucleic acids such as RNA. Once the sample enters the device, the nuclease enzymes are deactivated by heating and two chemicals. In the next step, the viral RNA is squeezed out, concentrated and transported through the saliva through a filter membrane. "This membrane was an essential component for collecting the nucleic acids and their concentration, a fact that increases the sensitivity of our method," said the lead researcher. In the next step, the RNA sample undergoes a freeze-drying process together with the CRISPR components to obtain a dried and inactive sample that can be activated at any desired time after that by puncturing sealed water vials inside the device. This reaction increases the amount of RNA in the sample and enables the detection of the desired RNA sequence. "Our goal was to develop a complete and complete diagnostic device that did not require additional equipment," explains the lead researcher. "Basically, the subject spits a saliva sample into the device and then you just press a button and get the answer within an hour."
The researchers first tested their device using human saliva mixed with synthetic sequences of the coronavirus, then using 50 samples taken from patients who were diagnosed as positive for the virus. The researchers found that their device was at least as accurate as the PCR method that exists today, which requires taking samples from the nose with a swab, and which takes more time and requires the use of more equipment to handle the sample to obtain the results. The device emits a fluorescent result visible to the naked eye. The researchers also developed an applet for the telephones, an applet capable of reading the results and sending them directly to public health departments for more effective follow-up. The researchers believe that the total cost of their device can be reduced to only 3-2 dollars. If the device receives the approval of the American Food and Drug Administration (FDA) and is produced on a large scale, they hope that their device can be used by people who wish to test themselves in their homes, or within health centers in areas that do not have access to the usual equipment for diagnosing the virus and its various variants. "The ability to identify and track the new versions of the original virus is an essential ability to achieve effective public health, however, unfortunately, the new versions can currently only be identified through genetic sequencing carried out in special epidemiological centers, which sometimes do not even exist in developing countries," said the lead researcher.

2 תגובות
There is a proven commercial device from Kodod Merad that performs a similar technology test and detects the presence of the virus within 45 seconds. What is the innovation in the device presented in the article?
The Israeli batm company already markets such a kit. So what's new here?