Amlek - Scientists have identified a new mechanism that slows down and may even stop the natural aging process of the cells of the immune system
We all know that as we age, it is easier to catch infectious diseases and they do more damage to the body. The reason is that the immune system itself ages, wears out and weakens, then outside pests manage to harm the body, and long-term inflammatory processes sabotage our health. Ideally we would like the cells of the immune system to be young and always ready for action, but wishes are separate and reality is separate.
And yet, the cells of the immune system live longer than we would expect from normal cells. They may not stay young forever, but they certainly manage to bypass the "Haiflick limit". And this is an unusual achievement at all.
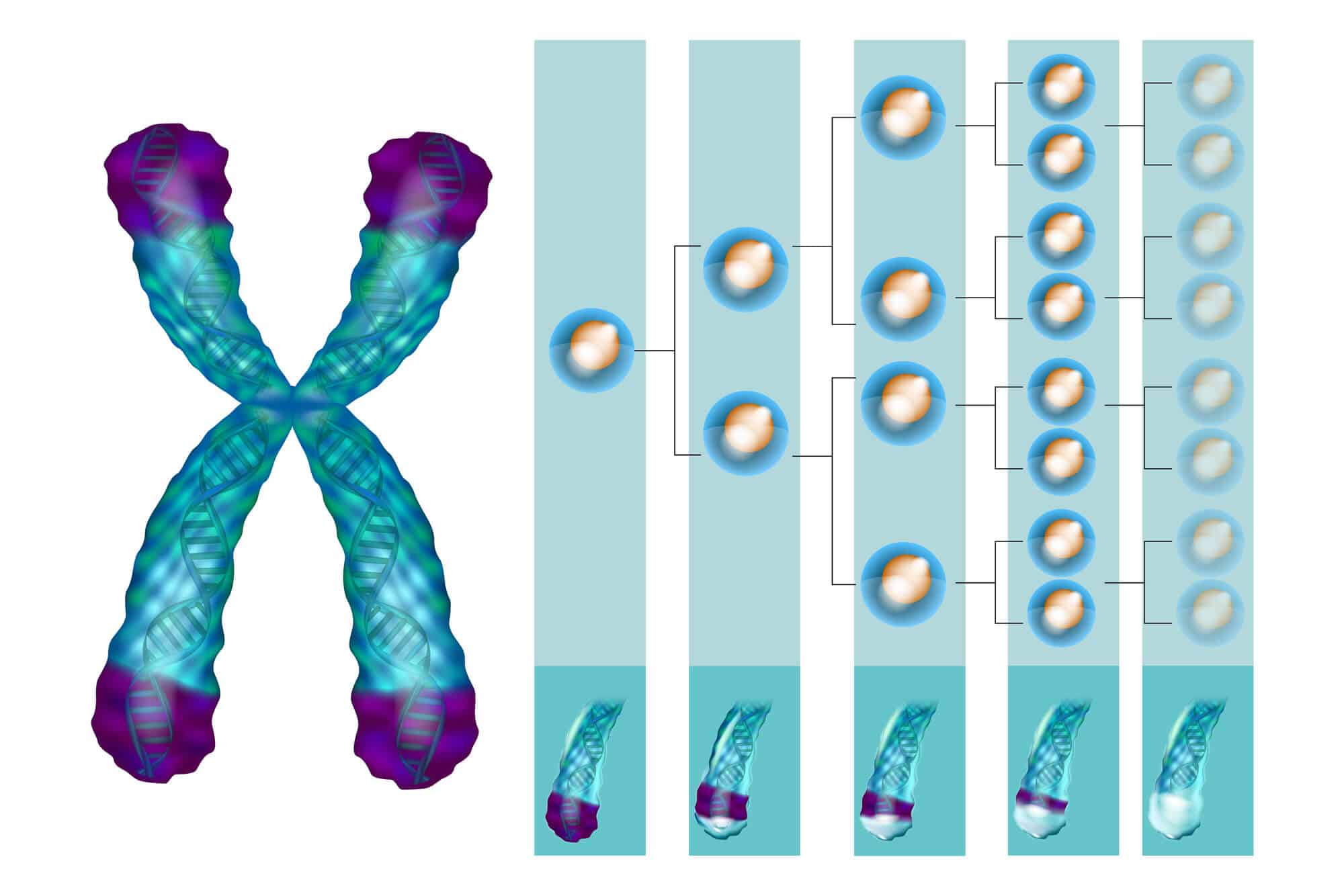
What is the Hayflick limit? It is a kind of stopwatch that works in the heart of every cell. Each time the cell divides into two new cells, the clock ticks once. In normal human cells, the clock counts only fifty ticks, and then is silent. Fifty ticks - fifty divisions: this is the Hayflick limit. When the cells reach this boundary line, they realize that they have reached the end of their journey. At this stage they commit suicide, or enter a coma and remain in the tissues as elderly cells that do not help the body.
How do cells know when they are approaching the Hayflick limit? How do they manage to count their number of divisions? The answer lies in special structures inside the cells called "telomeres". You can think of them as the plastic caps that cover the ends of your shoelaces. In our case, the shoelaces are the chromosomes, which contain the genetic code of the cell, and the telomeres are the stoppers that prevent the chromosomes from unraveling.
The telomeres themselves consist of DNA segments that repeat themselves thousands of times. Every time the cell divides, the telomeres get a little shorter. After about fifty divisions, the telomeres are already so short that the cell is unable to divide anymore. At this stage, as mentioned, he commits suicide or goes to the body's nursing home.
If you have now read this explanation for the first time - and understand it - you must have had a moment of enlightenment. "Eureka!" - you say - so this is the reason why we grow old! The cells divide, the telomeres shorten, and with every such shortening, we get older!
Unfortunately, this is not accurate. The fact that the telomeres shorten explains only a small part of the aging of the body, and is probably particularly relevant in the cells of the immune system. There are other mechanisms - which we will not go into here - that we suspect have a greater effect on aging. Telomere shortening is only one of many mechanisms.
And a new study published in recent months shows that even that we do not fully understand.
Telomerase is no longer alone
I wrote earlier that the white blood cells are able to bypass the Hayflick limit. They do this using an enzyme called telomerase. And it's not just the white blood cells that do this. For forty years we have known that this enzyme works in a very small part of the body's cells, giving them extraordinary longevity. But even in the cells where it works, it gradually stops functioning.
In the new study published a few months ago, the researchers identified a new mechanism for lengthening telomeres, which is not related to telomerase. They examined the way white blood cells (T lymphocytes) fight bacteria, and saw a strange process before their eyes: the transfer of telomeres. Other white blood cells came to the lymphocytes and sent them tiny bubbles: like battle rations we send to the soldiers at the front. The lymphocytes received their battle rations, opened them - and suddenly lengthened their telomeres. Accordingly, they were able to live longer and fought better against the invader over time.
What did those battle rations contain? telomeres. Simply, telomeres, which the lymphocytes have matched to their existing telomeres. As mentioned, we never knew that such trade in telomeres was even possible between the cells.
"The telomere replacement reaction between immune cells adds to the Nobel Prize-winning discovery of telomerase." explained Dr. Alessio Lana, who led the current study. "It shows that cells are able to exchange telomeres among themselves as a way to control the length of chromosomes before the action of telomerase begins. It may be possible to slow down or cure aging simply by replacing telomeres."
After the research group realized that they had a potential new mechanism for stopping aging - at least in one particular type of cell - they began to investigate it in earnest. The researchers were able to filter the 'battle rations' - the bubbles that contain the telomeres - from the blood of mice. They showed that when they deliver this extract themselves to lymphocytes, they 'restore their youth' in the immune systems of mice and are able to fight new invaders better and for longer.
what is the meaning? First of all, that we have a new direction to extend the life span of some white blood cells. Until now, we thought that only telomerase could do this, but it is difficult to induce it to act safely in cells. The new discovery could help us extend the lifespan of white blood cells - and perhaps of cells that we would like to treat in general - and thus delay some of the symptoms of aging, such as the weakening of the immune system.
Second, and perhaps more exciting at the concept level, I enjoy realizing - every year anew - how much we still don't know in the field of biology. If this discovery is true and cells really can exchange telomeres among themselves, then all textbooks on the immune system will include this information in the next edition. What other systems in our bodies remain somewhat unknown, waiting to be discovered? And what can we do with them when we decipher their existence?
It's exciting to think about the future - and the obvious fact that we still have so much to learn about it, and also about us.
The original article in Nature Cell
More of the topic in Hayadan:
