Weizmann Institute of Science scientists have uncovered a mechanism of the immune system that helps fight Candida infections
Of all the fungi that inhabit our bodies, perhaps the most notorious is Candida. This distant relative of baker's yeast is the most common cause of infections in the oral cavity, vagina and other organs, and sometimes even leads to life-threatening invasive infections. In a new study published today in the scientific journal Nature Immunology, revealed a group of scientists headed by Prof. Jacob Abramson From the Weizmann Institute of Science an unknown defense mechanism that our immune system activates in its war against the fungus. These findings may pave the way for new treatments for fungal infections.
Despite the bad name it has received, Candida is often part of the natural microbiome of healthy people - that is, it is part of the population of microorganisms that coexist with us on the skin or in the gut. In normal conditions, the levels of candida in the body remain extremely low thanks to the work of the immune system which prevents them from multiplying excessively. However, sometimes the balance is violated and the candida can get out of control, invade different tissues - and in extreme cases spread to the bloodstream and from there to the kidneys. These life-threatening infections are more common when the immune system is weakened, for example, in people taking drugs that suppress the immune system, such as chemotherapy or steroids. Antibiotics can also lead to a local or invasive outbreak of Candida, as their damage to the population of friendly bacteria in the body gives the fungus an unfair advantage in the power relations within the microbiome. This is, for example, the reason why women may develop a vaginal fungal infection after antibiotic treatment.
Until now, most of the credit for protection against candida has been given to a specific type of T cell, called TH17. However, in the new study, led by postdoctoral researcher Dr. Jan Dovash from Prof. Abramson's laboratory in the Department of Immunology and Biological Regeneration, the scientists showed that this elite patrol of T cells would not have formed at all without the operational backing of much simpler soldiers: a subtype of innate lymphoid cells called ILC3.
These two groups of cells belong to two separate fighting arms of the immune system - the innate arm and the acquired arm. The lymphoid cells on which the research was focused are part of the earlier immune arm, the innate one, which comes into action immediately upon the detection of some threat or in this case a fungal infection. The Candida-fighting T cells, on the other hand, belong to an evolutionarily later arm, the acquired arm, which needs a few days or even weeks to kick in, but is capable of delivering a much more powerful and focused blow than the innate arm.
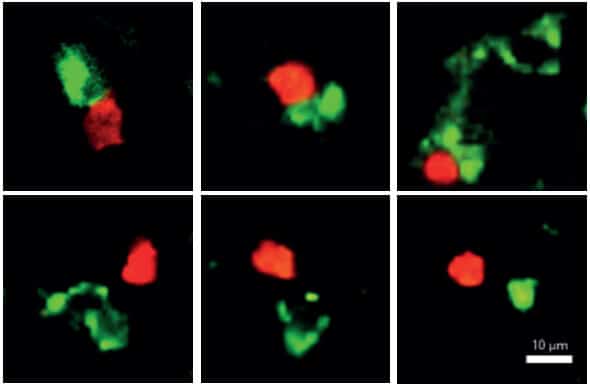
The scientists discovered that as soon as the candida gets out of control and begins to invade the body's tissues, soldiers of the innate immune system come into action and express a gene called AIRE - a gene that until now was mainly known for its involvement in the prevention of autoimmune diseases. These soldiers simply pounce on the mushrooms, swallow them whole, cut them into pieces and display the prey on their outer shell. This is how they signal the dedicated T cells, a small number of which are permanently on standby in the lymph nodes, to rapidly divide and assemble an elite patrol force of hundreds or even thousands of Candida fighters capable of effectively eliminating the fungal infection.
"We have uncovered a weapon of the immune system that was unknown until now and is essential for activating an appropriate immune response against a fungal infection," says Prof. Abramson who became interested in Candida following a mysterious phenomenon in patients with a rare autoimmune syndrome. Patients with this syndrome, caused by defects in the AIRE gene, develop, almost without exception, severe chronic candida infections. In previous studies in Prof. Abramson's laboratory and other research laboratories, it became clear that cells expressing the AIRE gene in the thymus gland "teach" the T cells of the immune system how to avoid attacking the body's own tissues. However, when there are defects in the gene, T cells do not receive the essential guidance, and therefore attack the body's tissues and cause great damage to many organs - as happens in that rare autoimmune syndrome. But why do the patients with this syndrome also develop a chronic fungal infection?
Dr. Dubesh and his colleagues found the beginning of the answer to the riddle outside the thymus gland: they discovered in the lymph nodes cells of the innate immune system that also express the AIRE gene. To test the involvement of these cells in the spread of Candida infections, the researchers created two experimental groups of transgenic mice: in the first experimental group, they silenced the gene in question in the thymus, and in the second group, they silenced it in the lymph nodes. The results were clear: the mice in the first group developed an autoimmune disease, but fought effectively against a fungal infection. In the second group, on the other hand, no autoimmune disease developed, but a significant fungal infection developed. In other words, they showed that without expression of the gene in the cells of the innate system in the lymph nodes, not enough dedicated T cells needed to fight Candida were formed. "In fact, we discovered a new function of the AIRE gene, which it performs in the lymph nodes - activating a mechanism that increases the number of T cells that fight Candida," explains Dr. Dubesh.
These findings open up new research directions that may allow the development of new treatments against Candida infections and possibly other fungal infections in the future. For example, it is possible to use the discovered mechanism to produce large amounts of Candida-fighting cells and inject them into patients as part of a "cell therapy" protocol. Moreover, further studies may reveal the specific molecular signals by which the cells of the innate system command the cells of the acquired system to proliferate. These signals themselves may be a target for the development of new drugs.
Osher Ben-Nun, Amit Binyamin, Dr. Liat Stoler-Barak, Dr. Yael Goldfarb, Dr. Noam Kadori, Yael Grofer, Tal Givoni and Ithi Zeliat from the Institute's Department of Immunology and Biological Regeneration also participated in the study; Prof. Ziv Shulman from the Department of Systemic Immunology of the Institute; Katarina Kobshova, Helena Bohmova and Yevgeny Welter from Charles University in Prague; Bergita Optdal and Prof. Eystein Husby from the University of Bergen in Norway; and Dr. Dominik Philip from the Institute of Molecular Genetics of the Czech Academy of Sciences, Prague.
More of the topic in Hayadan:
- Researchers from Ben-Gurion University have developed an experimental drug for psoriasis
- Researchers offer a new approach to dealing with the corona virus: putting the immune system into action even before the body is attacked
- There is no doubling of promotions: the immune system must choose - short term or long term
