The lethality of the malaria parasite lies not only in the strength of its attack on the body, but also in its softness
Red blood cells are our body's oxygen line, but they are also a perfect shelter for one of the deadliest creatures: the malaria parasite. About two weeks after entering the body, the Plasmodium falciparum parasite launches a massive attack: it quickly takes over a huge number of red blood cells and multiplies within them. At this stage the disease becomes life-threatening, and as evidence - every day about a thousand children die from it around the world. Two research groups at the Weizmann Institute of Science teamed up to find out what makes the invasion of the malaria parasite so effective - and discovered that The secret lies not only in the strength of the attack but also in its softness.
Dr Neta Regev-Rotsky And the members of her group in the Department of Biomolecular Sciences discovered that tiny bubbles secreted by the parasite in our body contain a surprising cargo: a proteasome - a molecular machine that breaks down damaged or unnecessary proteins. Her colleague in the department, Prof. Michal Sharon, specializes in proteasome research. In order to reveal what the proteasomes do in the malaria vesicles, the two research groups joined together, led by research student Elia Dekel from Dr. Regev-Rotsky's lab and post-doctoral researcher Dr. Dana Yaffe from Prof. Sharon's lab, and revealed how the malaria parasite uses the proteasome to to prepare the ground for the great invasion.
The parasite takes advantage of the flexibility of the red blood cells
Red blood cells have unique properties that make them particularly inviting hosts for the malaria parasite. First, in the process of their differentiation, they lose most of their cell organelles, thus losing their ability to defend themselves against harmful factors, including parasites. In addition, because by virtue of their role they have to reach the thinnest blood capillaries to supply oxygen to all body tissues, they have a tremendous structural capacity for flexibility; It is precisely this ability, it now turns out, that the parasite uses to its advantage.
After entering the human body, Plasmodium falciparum spends about two weeks in the liver, where it undergoes changes and multiplies. It then enters the bloodstream and invades mature red blood cells, and at the same time sends its neighbors a vanguard: vesicles carrying the proteasomes whose purpose is to soften these cells to facilitate penetration into them. This preparatory step allows thousands of parasites to invade a massive amount of red blood cells within seconds.
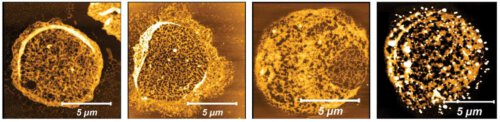
The researchers revealed how exactly the ground is prepared for invasion: they discovered that the bubbles secreted by the malaria parasite contain the version of the proteasome known as S20, which is smaller and needs less energy than other versions. In collaboration with Dr. Irit Goldian and Dr. Sidney Cohen from the Department of Infrastructure for Chemical Research, the research team developed an imaging method based on atomic force microscopy and revealed that the proteasome pierces holes in the skeleton of the red blood cells and breaks down protein fibers in their membranes; The scientists identified four red blood cell target proteins that the proteasome breaks down. In addition, in collaboration with Dr. Ido Ozeri from the Department of Life Science Research Infrastructures, they developed an artificial intelligence algorithm capable of identifying cellular skeletons softened using S20.
The relationship between the proteasome and cancer
To make sure that the proteasome is indeed responsible for the softening action, the scientists blocked its active site. This blockage prevented the softening of the cells, and accordingly severely limited the parasite's ability to invade them and its culture. This finding opens a new direction for the development of antimalarial drugs that will focus on blocking the parasite's proteasomes. The findings may also be relevant to other infectious diseases, since components of S20 have also been found in bubbles secreted by other parasites, for example, those that cause sleeping sickness and Jericho's rose. Moreover, this study may shed new light on cancer, since subunits of the proteasome S20 have recently been discovered also in tiny bubbles secreted by cancer-promoting cells in our body.

Dr. Gili Ben-Nisan, Dr. Yifat Ofir-Birin, Dr. Matia Morandi, Yael Ohana Daniel, Pula Abu Kerem, Daniel Alfendri, Shimarit Malihi, Tal Blok Tamin, Dr. Or-Yam Rozh also participated in the study. , Ariel Rodik and Dr. Uri Avinoam from the Department of Biomolecular Sciences; Dr. Ron Rothkopf and Dr. Ziv Porat from the Department of Life Sciences Research Infrastructures; Bakshi Molik and Prof. Nir Gov from the Department of Chemical and Biological Physics; Dr. Tamar Ziv from the Technion; Dr. Javier Cisquale, Dr. Matthew Pimentel, Dr. Thomas Navel and Dr. Eugene Kapp from the University of Melbourne and the Walter and Eliza Hall Institute for Medical Research; Julia Bergamashi and Prof. Chais White from the Free University of Amsterdam; Dr. Raya Sorkin from Tel Aviv University; and Dr. Teresa Carvalho from La Trobe University in Melbourne.
More of the topic in Hayadan:
