Hayadan > Corona vaccine
Corona vaccine
- Weizmann Institute
- March 3, 2022
Critically ill, recovering or vaccinated - how do the antibodies to corona that each of the groups produces differ and what are the unique characteristics of the antibodies acquired following vaccination
- Avi Blizovsky
- September 16, 2021
- 3 תגובות
This is according to a study by researchers from the Ministry of Health and the universities and hospitals in Israel that examined data on over one million Israelis aged sixty and over who were vaccinated with two vaccines at most in March 2021. The comparison was between those who received only the two original vaccine injections and those who also received the third vaccine
- The science service
- September 11, 2021
- No comments
- Weizmann Institute
- August 20, 2021
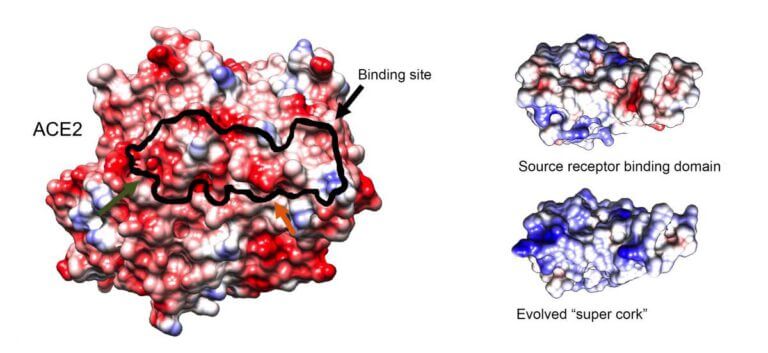
Through "evolution in a test tube" scientists of the Weizmann Institute of Science created a molecule that may be used as an effective cure for Corona
- Avi Blizovsky
- February 25, 2021
- No comments
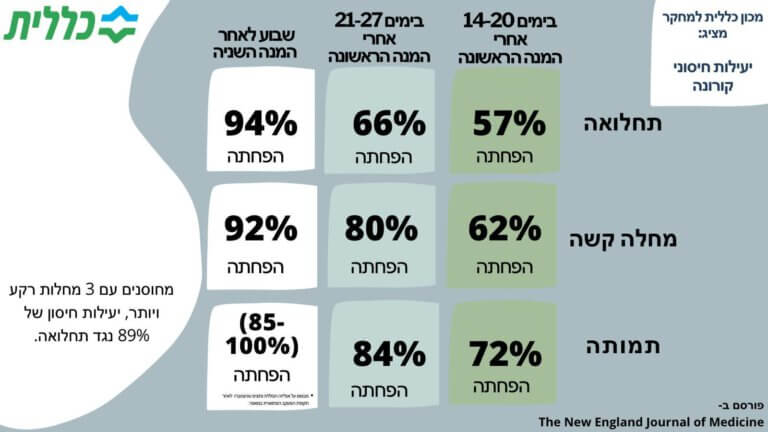
Final results of the Klalit Research Institute regarding the effectiveness of corona vaccines are published in the medical journal The New England Journal of Medicine: after the first vaccine dose: 57% effectiveness in preventing morbidity and a 62% reduction in severe disease after a second vaccine dose: a 94% reduction in morbidity and 92% are seriously ill
- Dr.Roey Tsezana
- February 17, 2021
A week ago a study was published in Nature in which the researchers described how they closely followed the evolution of the virus in the body of a cancer patient whose immune system was destroyed and discovered mutations that are also able to deal with a large number of antibodies produced against the virus
- Dr.Roey Tsezana
- January 21, 2021
In recent days there has been a big uproar regarding the Helsinki Committee, what it determined or did not determine, and what it means for the vaccine. So let's break down the issue, to understand what the Ministry of Health really did wrong, and what this means for Pfizer, the vaccine and the Helsinki Committee. Dr.Roey Tsezana makes order
- Avi Blizovsky
- January 15, 2021
- No comments
Dr. Borla will receive the title for his extraordinary achievement in leading the development of the innovative vaccine against SARS-CoV-2, the virus that causes the corona epidemic. The vaccine that helps in recovering from the corona crisis is expected to be a model for the development of a wide variety of mRNA-based treatments
- Avi Blizovsky
- December 20, 2020
- No comments
Moderna reported on Friday the receipt of FDA approval for the company's COVID-19 vaccine in the US, which will also be followed by approval for the vaccine in Israel as happened with Pfizer's vaccine last week
- Avi Blizovsky
- December 1, 2020
- One response
Moderna announces the analysis of preliminary efficacy findings in the results of the phase 3 trial of its COVE vaccine for the treatment of COVID-19 as well as the submission of the trial findings for emergency approval by the FDA
- Avi Blizovsky
- November 16, 2020
- No comments
A first interim analysis of the experiment's findings included 95 participants who were infected with Corona, 90 of whom are from the control group * All five who got sick despite the vaccine were asymptomatic or in a mild condition * Israel is among the first in line when the vaccine is approved
- The science service
- November 11, 2020
- 7 תגובות
A new study conducted at the Safed Academic College reveals that the more children are vaccinated in the general population in a certain country, then the death rates among those infected with the corona in that country are lower
- Avi Blizovsky
- November 10, 2020
- One response
This follows an interim analysis of a clinical trial that is in progress, in which it was found that out of 94 confirmed corona cases among the experimenters, only nine received the vaccine and the rest are from the control group that received a placebo
- Avi Blizovsky
- September 28, 2020
- 5 תגובות
This is a vaccine based on Jensen's Ebola vaccine. "Four vaccines in clinical trials is an unprecedented achievement by the scientific community made possible by decades of advances in vaccine technology and a coordinated strategic approach between government, industry and academia," said NIAID Director Anthony Fauci.
- Avi Blizovsky
- September 15, 2020
- No comments
- Dr.Roey Tsezana
- July 8, 2020
- 20 תגובות
- Avi Blizovsky
- May 20, 2020
- 5 תגובות
- Science site The Conversation
- May 10, 2020
- 10 תגובות