Israeli research strengthens the hypothesis that the cause of diseases such as Alzheimer's, Parkinson's and diabetes in adults is not amyloid deposits, but round protein structures that appear before the formation of the deposits
In diseases that erupt in old age, such as Alzheimer's, Parkinson's, Creutzfeldt-Jakob, adult diabetes and more, deposits appear that look almost identical under an electron microscope. In 1901, the American doctor Eugene Opie discovered these deposits in the pancreatic cells of diabetic patients who had died, and in 1906, the German doctor Alois Alzheimer discovered them in the brain of an elderly patient suffering from dementia.
The deposits were named amyloids, because, like starch, they were colored when iodine was added to them. The amyloid deposits are composed of proteins. Proteins consist of long chains of amino acids, which can fold into a very large number of three-dimensional structures. The accepted assumption is that the misfolding of proteins and their association together to form precipitates is the basis of the amyloid diseases, which are otherwise unrelated.
Since it is assumed that the sediments are responsible for the development of diseases, research in the field has focused on trying to break them down. However, the findings in the field did not always match the theory. There have been cases where post-mortem examinations have found large amounts of amyloid deposits in the brains of people who did not suffer from behavioral symptoms of the diseases.
One of the prominent examples is Sister Mary, a nun who died at the age of 102 and donated her body to science. Until the day she died, Sister Mary was lucid and vital, but in the post-mortem examination it was found that her brain was full of amyloid deposits. This and other findings raised the suspicion that the damage of the diseases is not caused by the amyloid deposits but by other substances.
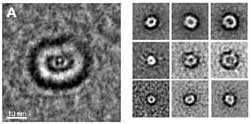
About two years ago, Peter Lansbury from Harvard University hypothesized that the causes of amyloid diseases are protein structures that appear before the formation of the deposits. Lansbury identified circular structures resembling donuts, which appeared in the early stages of the creation of the proteins associated with Alzheimer's and Parkinson's. He called these structures "amyloid pores".
Dr. Ehud Gazit from the Department of Molecular Biology and Biotechnology at Tel Aviv University decided to check if the "doughnuts" identified by Lansbury also appear in other amyloid diseases. Gazit focused on adult diabetes (type 2 diabetes), one of the most common amyloid diseases. During the disease, the body gradually stops responding to insulin and needs increasing amounts of it, in order to inject sugar into the cells. At the same time, large amounts of the protein amylin are secreted, which creates amyloid deposits that cause the destruction of the pancreas.
"The names of the cells are known to be caused by holes that form in their outer membrane," says Gazit. "We tried to understand which of the structures formed from the amylin protein is responsible for this injury."
To this end, Gazit and research student Yair Porat used a method developed by Dr. Raz Yelink and Dr. Sofia Kolusheva from Ben-Gurion University. The two researchers developed an artificial cell membrane, which acts similarly to the natural membrane and allows the binding of proteins to it to be monitored.
Gazit and Porat placed a solution containing human amylin protein under conditions where the protein forms the amyloid structures. After that, two segments were separated from the solution: a liver segment that sank in a test tube, where amyloid deposits were found; and a soluble section, which contained amylin proteins that did not have time to aggregate together and small aggregations of proteins that did not yet form precipitates.
In the next step, the researchers tested the ability of the two segments to bind to the cell membrane. "It became clear to us that the main action of the link was in the soluble section," says Gazit. "We discovered in this section a factor that damages the membrane and disappears over time. This active factor is not found at the beginning of the experiment - it appears after a while and then disappears. Therefore, it was clear that this was a small factor that was built and then decomposed, and not the protein itself."
The next step was to trace with an electron microscope the structures that form in the two sections. "We saw that during the peak of the binding to the artificial membrane, circular donut-like structures appear in the soluble section, which are strikingly similar to those Peter Lansbury identified in Alzheimer's and Parkinson's," says Gazit. "Furthermore, examination with an electron microscope revealed that while the section containing the donut-like structures caused a complete breakdown of the membranous structure, the amyloid deposits did not cause any damage to it. The main conclusion from this is that the main harmful factor is not the protein deposits, but the structures that form before it." The study was published in the September issue of the journal Biochemistry".
According to Gazit, the findings emphasize the need to shift the research emphasis from breaking down the sediments to preventing their creation in the early stages of diabetes, when the donut-like structures appear. He even showed in preliminary experiments that small ring molecules inhibit the process leading to the formation of the precipitate. These experiments strengthen the approach according to which inhibiting the creation of the donut-like structures may prevent the development of diabetes.
