7Apheresis combines a drug and a device with a dry formulation of human insulin that is secreted through a small, portable inhaler in order to help patients achieve sugar balance. Apreza is quickly absorbed and has a short duration of action. It was taken at the beginning of the meal.
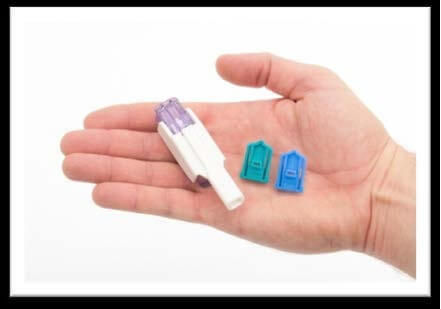
Sanofi and MannKind announced today that Afraza (insulin human), the only inhaled insulin, is now marketed by prescription in pharmaceutical chains across the US. Apreza is approved by the US Food and Drug Administration (FDA) to control high blood sugar levels in adults with type 1 and type 2 diabetes.
"Many people living with diabetes are unable to achieve balance with the medications they take and may need to use insulin. Now, they have another option for taking insulin other than by injection," said Dr. Janet McGill, professor of medicine at the Washington University School of Medicine. Lewis and a clinical researcher of apheresis. "This option of taking may change the dialogue between the treating medical team and people living with diabetes when it is necessary to start insulin treatment or its intensification."
Apreza combines a drug and a device with a dry formulation of human insulin that is secreted through a small, portable inhaler in order to help patients achieve sugar balance. Apreza is quickly absorbed and has a short duration of action. It was taken at the beginning of the meal.
Apreza can help balance high sugar levels as part of a diabetes management plan that may include strict diet, exercise and treatment with other diabetes medications.
Patients with chronic lung diseases, such as asthma and COPD, cannot take apheresis. Also, it cannot be used as a treatment for diabetic ketoacidosis. Apreza is not recommended for patients who smoke or who have recently stopped smoking.
"Afreza is an important addition to Sanofi's expanding portfolio of integrated and customized diabetes treatment solutions. It underlines our commitment to bring innovative treatments for people with diabetes," said Pierre Chansel, senior vice president, head of Sanofi's diabetes division. "There is a clear need for insulin that does not require an injection and Sanofi is committed to bringing this therapeutic option to the well-being of patients."
According to Alfred Mann, chairman of the MannKind company: "We are especially proud that we have reached this day when Apreza, which is the product of many years of development, is accessible with the aim of helping people manage diabetes."
Roni Birnbaum, CEO of Sanofi Israel: "This is real good news for diabetics all over the world. Sanofi Israel will make every effort to bring AFREZZA to the Israeli market as soon as possible for the benefit of the patients."
