Researchers discovered that when helium is cooled almost to absolute zero, compressed and made to oscillate around an axis - it behaves in a way that has never been observed before
Kenneth Chang New York Times, Haaretz
Quantum mechanics has done it again. Researchers at Pennsylvania State University in the US performed an experiment with extremely cooled helium, and discovered that when a solid cylinder rotates around its axis back and forth - part of it may not move at all.
At extremely low temperatures, matter often behaves very differently from what we are familiar with under normal conditions. For example, many materials become superconductors, capable of conducting electricity without any resistance, because the electrons connect and move in a coordinated manner without damaging the lattice of atoms that surrounds them.
Similarly, other materials at low temperatures become a superfluid, moving without any friction due to the lack of viscosity to slow its speed. The new experiment suggests another mode of aggregation: supersolid.
In the seventies, physicists toyed with the idea that a solid - a substance whose atoms are organized in an ordered crystalline structure - might undergo a quantum conversion (change of state). Some claimed that when a solid is cooled to a sufficiently low temperature, some of its atoms will "melt" and become a superfluid, which will flow effortlessly through the solid material surrounding it. They called this state "super-solid".
Most physicists, including those who proposed the idea, have come to the conclusion that although this situation is possible, the chances that it will be possible to observe it are very small.
"I wrote at least one paper in the distant past about the possibility of supersolid behavior," said Dr. Anthony Leggett, a professor of physics at the University of Illinois, who received the Nobel Prize in Physics last year for his theoretical research on the superfluid. "I would bet at least a hundred to one against such a possibility."
But in an article published this month on the website of the scientific journal "Science," Dr. Moses Chan, a professor of physics at Pennsylvania State University, and Dr. Yunsung Kim, a postdoctoral student who works with him, reported that they succeeded in turning helium into a supersolid. "Theorists will be surprised," said Dr. Chan, "but they are not ruling out this possibility."
Helium, the same gas that allows airships to lift a chicken and makes those who swallow it sound like Donald Duck, turns into a liquid at a temperature of minus 269 degrees Celsius. If you cool it a little more and compress it under enormous pressure, the helium atoms are pushed together and become a solid.
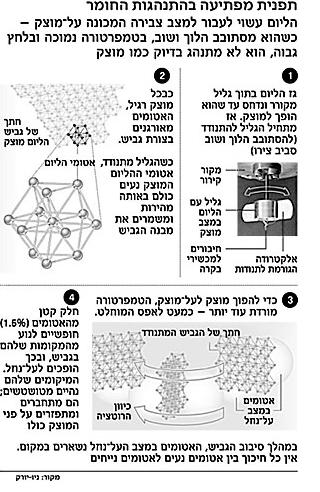
In the experiment, Dr. Kim and Dr. Chan placed helium in a small cylinder, cooled it to almost absolute zero (minus about 273 degrees Celsius) and applied pressure ranging from about 26.5 kg per square cm to about 67.5 kg c per square cm (the normal atmospheric pressure on the surface of the earth is XNUMX kg per square cm), until it became solid.
The scientists turned and released the cylinder, which was attached to the rod. The twisting force of the rod caused the cylinder to oscillate (spin back and forth around its axis) at a constant frequency, determined by the mass and dimensions of the cylinder.
When they continued to cool the cylinder down to a temperature of minus 272.9 degrees - about a tenth of a degree above absolute zero - the frequency of oscillations increased, as if the ring was losing its mass, until it seemed as if 1.5% of the helium had disappeared.
The scientists from Pennsylvania believe that 1.5% of the solid helium has become a superfluid, and in accordance with the laws of quantum mechanics have moved to the most "lazy" energetic state: immobility. While the solid cylinder oscillated back and forth, the superfluid remained in place in a way that appeared to flow in the opposite direction.
To make sure that some of the helium really hadn't evaporated, the scientists reheated the cylinder and the oscillations slowed back down. They also repeated the experiment with a lighter version of helium, helium-3, which is known to not become a superfluid, and found no change in the frequency of the oscillations.
So far, other scientists agree that the "supersolid" explanation is the most logical, but they want to examine more data. "I think everyone agrees that more experiments must be done," said Dr. Wayne Saslow, a professor of physics at Texas A&M University.
There is no ready answer to the question of how a superfluid can flow through a solid. "I think this is a big puzzle," said Dr. Leggett. "If this finally turns out to be true, it will cause us to completely re-examine our perception of exactly what a solid is according to quantum mechanics."
One of the possibilities, said Dr. Leggett, is that the helium solid is not perfect - that several places in its crystal are empty and the superfluid flows from one empty site to another. Against this explanation stands the finding that higher pressures, which are supposed to push atoms to fill those empty spaces, did not detract from the supersolid phenomenon.