The constant search for weak points of the AIDS virus yields new ideas for drugs from a new family
By Gary Stix, Scientific American.
The article appeared in Issue No. 25 of the journal, October-November 2006
Virus researchers invest a significant portion of their resources in examining every tiny step in the life cycle of the AIDS virus (HIV) - starting with its attachment to a cell from the cells of the immune system and its entry into it, to its replication, to the release of new viruses from the host cell and the search for a new victim cell. The last important family of drugs in the fight against HIV was introduced about ten years ago with the discovery of protease inhibitors. Protease is an enzyme that breaks down proteins, and the inhibitors disrupt its vital action for the last step in virus replication.
At the time, some members of the AIDS research community wondered if protease inhibitors could serve as the basis for a drug. But hopes were dashed in view of the cunningness of the virus. In one of the studies it was discovered that up to half of the AIDS carriers receiving treatment in the US are infected with viruses that have developed resistance to at least one of the drugs they are taking. The clinical experts can choose from more than 20 protease inhibitor drugs and two families of drugs that prevent the invading virus from replicating its RNA into DNA, thus sabotaging its ability to replicate. Giving a combination of these substances (the "cocktail") to patients is supposed to work against the inherent ability of the virus to mutate. But this approach does not always prevent the development of drug resistance, including resistance to protease inhibitors. "Given the growing resistance to protease inhibitors, it is of great importance to find new ways to disrupt the replication cycle of the virus," says Eric Freed, a researcher in the AIDS Drug Resistance Research Program at the US Institutes of Health (NIH).
At the moment there are substances in various stages of development that interfere with the viral processes inside the host. These substances disrupt the processes at the beginning, in the middle or at the end. Researchers at an academy and a small biotechnology company called Pancos in Watertown, Massachusetts are working inspired by the success of protease inhibitors to develop new substances, called "virus maturation inhibitors", that block protease activity in a new way. Protease inhibitors directly attack the HIV protease, an attack that prevents the enzyme from processing a viral protein called GAG. When GAG proteins undergo appropriate cutting, the parts derived from them form the conical core, called the capsid, which surrounds the RNA and protects it. In contrast, Pancas' maturation inhibitor blocks a site on the GAG protein that the protease normally binds to. This prevents the protease from cutting the GAG protein correctly. As a result the box is not built properly and the virus cannot infect another cell.
look for clues
The path to Pankus' intended drug began in the mid-90s, when the Boston Biomedica company began collaborating with a professor from the University of North Carolina at Chapel Hill. The partners sought to screen compounds found in a collection of traditional Chinese medicinal herbs for biochemical activity against HIV. Kyu-Hsiung Lee's lab discovered a possible drug candidate in a Taiwanese medicinal herb.
The compound found, betulinic acid, showed weak activity against HIV. After the laboratory analyzed its chemical composition, it became clear that after a chemical change a derivative with a much stronger effect is obtained. "Betulinic acid was active against HIV at micromolar concentrations," says Graham Alavi, chief operating officer of Pancos. "This derivative worked at nanomolar concentrations."
Six years ago, Boston Biomedica spun off its HIV research unit and founded Pancus, which began researching the compound, designated PA-457. This time it was not a new case of Taxol, the anti-cancer drug whose production required the controversial cutting down of rare yew trees until a semi-synthetic substitute was found. Pancus did not need a steady supply of Taiwanese herbs. The betulinic acid can be extracted from dolav trees and common alba trees. Another step of chemical processing yielded the desired molecule.
Although the researchers realized that PA-457 works against all strains of HIV, they had to find out how the betulinic acid derivative works against the virus at the molecular level. The company wanted a new class of drug, not another protease inhibitor. She therefore contacted Freed's lab at the NIH, which was studying the life cycle of the virus.
Fried's research group and the researchers at Pankos determined that the drug works in the late stages of the viral replication process, probably during the box formation stage. Researchers already knew that the HIV box is formed when newly formed GAG proteins bind to the inside of the host T cell membrane. Then they are cut by the HIV protease into short pieces. They also learned during the development process of the protease inhibitors that any disruption in GAG processing would render the virus non-infectious. And so they began to study the interaction between PA-457 and GAG proteins, to find out exactly how the compound disrupts the cutting of GAG into the parts needed to build the box.
Cultivating resilience
The first step in understanding how a particular compound works is often creating resistance. This allows scientists to identify the exact point where the drug interacts with its site of action. To foster resistance, Farid and colleagues delivered low-dose PA-457 to a culture of HIV-infected T cells. Then the genetic sequence of the resistant viruses was determined and compared to the sequence of the viruses that succumbed to the drug. This test makes it possible to locate the site that has undergone a change in the resistant viruses that have been created. It turned out that this site is in the GAG protein, where it binds to the protease. This change prevented PA-457 from blocking the enzyme's activity.
Analysis of the resistant strains allowed the researchers to confirm that PA-457 was no longer just a protease inhibitor. Most drugs, not just HIV inhibitors, work by binding to enzymes. "An action directed at the substrate [rather than the enzyme] is an unfamiliar and surprising action," Alavi says. "As a result, we believe we will be in a fairly strong position to obtain a patent."
Cultivating the resistant strains does not necessarily mean that the drug is expected to have a short medical lifespan. In fact, resistance to PA-457 may not develop rapidly because the binding site on the GAG proteins to which it binds is similar in different HIV strains, and therefore not easily altered by mutations.
The drug PA-457 has already passed an intermediate stage of clinical trials that tested its activity in patients who took it for 10 days compared to another group that took a dummy drug (placebo). AIDS viruses replicate themselves so quickly that a short experiment is enough to determine if the drug attacks the pathogen in the body. On average, there was a 92% decrease in the level of the virus at the highest dose of 200 milligrams. The goal of the study was to achieve a decrease of at least 70% in the index called viral load, as an initial sign that the drug is effective. However, some patients did not respond, and the company will determine in the next testing phase if it is possible to give higher doses than that. "The main message is that this is an active drug and that the research should continue," says Jeffrey M. Jacobson, who is in charge of the subject of infectious diseases at the Drexel University College of Medicine and the principal investigator of the clinical trials.
In the next round, the researchers will test interdrug activity with other drugs, a necessary requirement for testing an anti-HIV drug, since AIDS treatment must include more than one drug due to the fear of resistance. These days the US Food and Drug Administration (FDA) encourages these tests to be conducted in the early stages of clinical trials. During the development of new AIDS drugs in the past, researchers discovered drug-drug interactions only at much later stages in the clinical testing process. If all goes as planned, Pankos could seek final FDA approval in 2008.
Additional ripening inhibitors
PA-457 is not the only example of ripening inhibitors, although it is the most advanced in the commercial race. Independently, researchers at the University of Alabama and the University of Maryland discovered small organic molecules that prevent the many subunits of the box from joining together to form the final envelope. "We're trying to change the pieces in such a way that they can't fit together," says Peter Provelige, a professor in the Department of Microbiology at the University of Alabama.
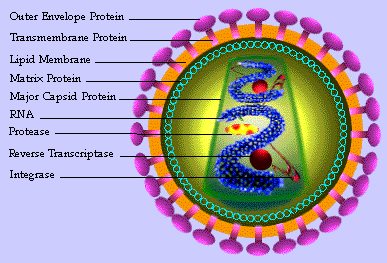
This approach is progressing in parallel with other approaches in development that are designed to sabotage the life cycle of the virus. Entry inhibitors, including a substance that Pankos is also researching, prevent the virus from entering the cell. (One entry inhibitor, given by injection, has already received FDA approval, but Pankos' drug is supposed to be taken by mouth.) Among the new drug families that have reached the advanced stages of trials are the integration inhibitors. These substances damage the enzyme that allows the DNA created by the virus to integrate into the DNA of the host cell and thereby prevent the production of new viral RNA. All these biological agents and more are needed. In the absence of a vaccine, which is not even in sight, this inferior virus - a capsule several nanometers long containing single-stranded RNA - will continue to outwit even the best ideas molecular biologists can come up with.
Maturation inhibitors are a new family of anti-AIDS drugs currently being studied. They attack the virus at a late stage of its life cycle - when components of the virus that have just been formed join together to form new infectious units that begin to "hawk" out of an infected T-cell so that they can continue to infect other cells.
normal maturation of
HIV
A single, complete HIV virus emerges from a T-cell wrapped in a layer taken from the host cell membrane and on its surface viral proteins are displayed. The virus's protease enzymes work to chop up the GAG protein molecules to make other, smaller proteins.
The box proteins unite to form a conical core, which together with the inner nuclear box seals and correctly places the viral genes, which are found in two molecules of single-stranded RNA.
treated virus
The designated drug, PA-
457, attaches to the GAG protein and prevents the protease from separating the box protein from the neighboring protein in the GAG - the SP1 protein.
As a result, the hybrid structure formed from box-SP1 and box proteins
The internal ones get an abnormal shape. This probably prevents the virus from replicating itself properly.
And more on the subject
PA-457: A Potent HIV Inhibitor That Disrupts Core Condensation by Targeting a Late Step in Gag Processing. F. Li et al. in Proceedings of the National Academy of Sciences USA, Vol. 100, no. 23, pages 13555–13560; November 11, 2003.
The Prevalence of Antiretroviral Drug Resistance in the United States. Douglas D. Richman et al. in AIDS, Vol. 18, no. 10, pages 1393–1401; July 2, 2004.
The Discovery of a Class of Novel HIV-1 Maturation Inhibitors and Their Potential in the Therapy of HIV. Donglei Yu et al. in Expert Opinion on Investigational Drugs, Vol. 14, no. 6, pages 681–693; June 2005.

One response
Amazing... but it's really impossible to work on a real vaccine, or maybe it's just more financially profitable to constantly release new inhibitors?