This time we will deal with Pasteur's first discovery - the science of crystallography
Pasteur made his first major discovery as a doctoral student in chemistry, when he was immersed in the study of crystal science. His research focused on the formation of various compounds, and he began working with tartaric acid, which was discovered in crystalline form and in large quantities in sediments left at the bottom of wine fermentation tanks. Aside from the tartaric acid crystals, much smaller amounts of other crystals could be found inside the same containers. Those crystals were made of para-tartaric (or 'racemic') acid. The two different acids were discovered and studied several years before Pasteur's research began, and the scientists who studied them were able to prove that the crystals of the acids were identical to each other in every possible respect, except one. Although they looked the same under the microscope lens, and their chemical properties were also the same, but when polarized light was passed through a bottle containing diluted tartaric acid, the light was shifted to the right. When polarized light was passed through a bottle containing diluted paratartaric acid, the light was not deflected
This mystery puzzled Pasteur. How is it possible that there are two crystals that are identical to each other in every possible respect, but they react to light differently?
Pasteur's discovery finally came from careful observation of the various crystals through the microscope. Although the crystals looked the same at first glance, a closer look revealed that the paratartaric acid is made up of two different types of crystals, which are reversed in direction. Although both looked the same, one was the opposite, or 'mirror image', of the other. These crystals are called chiral crystals.
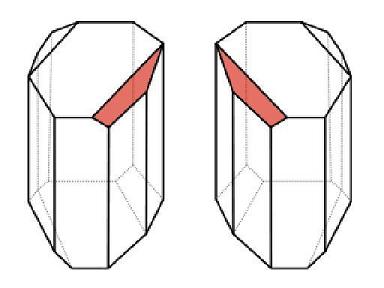
When we have a solution that consists only of chiral molecules of one type, such as tartaric acid, the polarized light we pass through it will be deflected to the right. But what will happen when we have a solution consisting of an equal number of chiral molecules of one type, and an equal number of chiral molecules of the second type? In this case, half of the molecules will deflect the light to the right, and the other half will deflect the light to the left. The two deviations cancel each other out, and in the end the light passing through the solution will not change its course at all!
Pasteur was able to differentiate between the two types of chiral crystals under the microscope lens, and realized that the paratartaric acid consists of a mixture in equal proportions of the different crystals, therefore it does not polarize light. In order to prove the theory, Pasteur undertook an operation of ant work. He dispersed crystals of paratartaric acid under the microscope lens and with the help of a sharp needle worked to separate the 'right' chiral crystals from the 'left'. When he finished, he had two piles of crystals in his hands. He dissolved each of them in a different bottle, thus proving that the paratartaric acid can be separated into crystals that polarize light to the right and crystals that polarize light to the left. A discovery of this magnitude required particularly strong proof, and Pasteur decided to invite Jacques Biot, the French expert on polarized light, and repeat the experiment in front of his eyes. Biot followed all the stages of the experiment and was amazed at the surprising result. After the article describing the results of the experiment was published, Biot gave a goshpenka to the article and expressed his support for Pasteur. The chemist community in France immediately understood the importance of the discovery and Pasteur became the second of the most popular scientists in France, at the age of 26. For discovering the science of stereochemistry, he was awarded a knighthood in the French Legion of Honor, and even the British Royal Society awarded him the prestigious Copley Medal.
Although the discovery may not impress us today, in Pasteur's time it was a conceptual breakthrough for the science of chemistry. We know today that most biological molecules are chiral, and in some cases a pair of molecules that look identical can have a very different effect on the human body due to their chirality. An example of this is the molecule aspartame, known as an artificial sweetener, but the taste of its chiral partner is bitter.
A more tragic example is the drug thalidomide, which was sold in Europe in the middle of the 20th century as an anti-nausea drug. Thalidomide consists of two chiral molecules, one of which prevents morning sickness - a common phenomenon in the first months of pregnancy. Many women took the drug, without being aware of the chiral partner of that molecule and its effect. The chiral partner of thalidomide causes defects in the development of the fetus and the birth of babies with missing limbs, or no limbs at all. Although the medicine bottle contained only thalidomide molecules with the correct chirality, the human body is able to reverse the orientation of the thalidomide molecules, thus creating the chiral partner, which harms the fetus. Over 50,000 people were affected by thalidomide, before researchers deciphered the link between the drug and the proliferation of fetuses with deformed, short and missing limbs [A].
From chiral molecules... to the whole world!
Based on the enormous popularity he gained in France, Pasteur could choose the university where he would continue his research. He decided to accept a position as professor of chemistry at the University of Strasbourg, where he continued to study the chirality of crystals. He discovered that substances that are not organic - that is, that do not originate from living things - are also not chiral. These substances are identical mixtures of the two types of chiral molecules, and therefore do not deflect the polarized light. In contrast, organic substances - and especially amino acids, which are the building blocks of all proteins - appear in only one chiral version in living things, and therefore deflect the polarized light. When Pasteur tried to prove his hypothesis, luck knocked on his door. He discovered a container of paratartaric acid with mold growing on it. When Pasteur tested the same acid, he discovered to his surprise that it contained only one chiral version of crystals, instead of the two versions that should be present in paratartaric acid. In further experiments, Pasteur realized that the mold consists of living creatures that feed on the 'left' chiral version of the crystals, and produce 'right' chiral crystals from it.
Between discoveries, Pasteur also discovered the daughter of the president of the University of Strasbourg, Marie Laurent. After several weeks of feverish courtship, Marie succumbed to the young, charismatic and abject scientist and they were married in no time. Of all his discoveries, some say that Marie Laurent was the most important of all later in his life. She was his companion and helped against him, and many times they would spend the evenings together, as he dictated letters and work protocols to her. Even when Pasteur suffered a stroke at the age of 45, which left his left leg and arm paralyzed for the rest of his life, Marie continued to support him in everything, and with her help he recovered and returned to active research. Marie and Louis Pasteur had five children, but only two of them reached full adulthood - a fact that is not surprising considering the sanitary and medical conditions of that time.
Immediately after his marriage, Pasteur returned to the study of chiral crystals. He strove to find the connection between the lack of symmetry - the fact that organic life is based on chiral molecules only - and the other forces that exist in the world. As can be read in Pasteur's writings:
"The whole universe is asymmetric. I tend to think that life, as we know it, is a product of the asymmetry of the universe and the results that flow from it. The universe itself is asymmetric... even the movement of sunlight is asymmetric... the magnetism of the earth, the contrast that exists between the south and north poles of a magnet and between negative and positive electricity - all these are but the results of asymmetric actions and movements... life is controlled by -by asymmetric operations. I can even imagine that all living things are fundamentally a result of cosmic asymmetry.” [B]
Pasteur began planning experiments that would show how a magnetic field opposite to that of the Earth affects plants, or how sunlight affects a plant when it rises in the west and sets in the east. His friends begged him not to waste his time on useless research, but Pasteur believed with all his heart in the correctness of his ideas. His wife wrote to Pasteur's father at the time that, "Louis puts too much time and thought into his experiments, but if they succeed they will give us a new Newton or Galileo." [C]
Despite his belief, Pasteur was unable to prove his ideas that the asymmetry of the universe is expressed in the asymmetry of living matter. Throughout the rest of his life he continued to adhere to the idea and publicize it, but was never able to discover solid proof of its truth. Pasteur, like Einstein, was a brilliant man. But like Einstein, he too could be wrong and believe in theories that failed to survive the sharp surgeon's knife of science.
In the rest of the article, we will review the experiments in which Louis Pasteur first became acquainted with bacteria and fungi, and how he used the knowledge he acquired to formulate his opinion about the spontaneous formation and make France a wine powerhouse in Europe.
A. Quoted in Dubos 1950, p. 111
B. Pasteur, Correspondence I, 10 Nov. 1853, p. 324
C. Pasteur, Correspondence I, 10 Dec. 1856, p. 412
Part I of the article
Part III of the article
Part D of the article
