In the current article, I want to go over the way in which the Pfizer and Moderna vaccines were developed, how they received emergency approval, and what this means in the end for their level of safety and effectiveness. To do this, we will go through each step in a typical vaccine development and approval process, and see how it went over the past year
From the moment the vaccines received FDA approval, questions began to rise throughout the social network: Are they really approved for use?
The answer is yes: they were approved. And no, they were not approved.
An order was made.
The reason for the confusion comes from the fact that the FDA uses two different terms in English, which in Hebrew sound the same. The first is Approval. From now on we will call it "regular approval". If the FDA has decided that the product is effective and safe to use - then it receives normal approval[I]. The process for obtaining such a permit is extremely long, and can easily reach 245 days or even more[ii].
The second type of authorization is called Authorization in English. Or in its full name - Emergency Use Authorization (EUA). We will call it "emergency approval" from now on. The FDA provides emergency approvals in exceptional situations, in which there is an urgent need for drugs or medical devices that can save lives, and the risk associated with their use is negligible. A situation, for example, in which a new virus circulates in the world, kills millions of people, causes side effects that we do not understand and along the way also causes the paralysis of entire countries[iii].
To date, vaccine companies have only received emergency approval from the FDA. And despite the fact that it is not a regular approval, the sweeping recommendation for all adults (apart from rare exceptions) is to use the vaccine without thinking twice.
Here the skeptics come up and ask - with absolute justice - how is it? After all, the development process of a normal vaccine can easily take 10-15 years[iv]. And while there have been some rare exceptions over the years—vaccines for Asian flu, swine flu, and Hong Kong flu that were developed in six months or less—they were approved quickly precisely because there was great fear of a global pandemic.[v]. These are not exactly the examples we would like to rely on, if we wanted to claim that the corona vaccine is safe and effective.
In the current article, I want to go over the way in which the Pfizer and Moderna vaccines were developed, how they received emergency approval, and what this means in the end for their level of safety and effectiveness. To do this we will go through each step in a typical vaccine development and approval process, and see how it has played out over the past year.
step one: Research
At this stage, researchers in the laboratories have to identify a certain substance that may have vaccine potential, characterize it, understand how it works and convince the managers or the pharmaceutical companies that it is worth taking the risk and trying to get approval for it from the FDA - a process that requires tens of millions of dollars. In total, this phase lasts between two and four years.
When companies tried to produce vaccines in the past, they had to isolate the viruses, then kill them - or at least weaken them greatly. In this way, the body would develop immunity against them, without the viruses being able to harm it. The trouble is that if we just kill the virus before injecting it into the body, then it may break down - and then the body will not develop immunity against it. And if we don't weaken the virus enough, then it will cause the disease itself. Weakening viruses is an art in itself, and can take many years. And if you have already succeeded in weakening the virus and injecting it into the body, then the immune system will develop hundreds and thousands of antibodies against all the areas on the surface of the virus that it recognizes. One of those antibodies may mistakenly recognize human cells as enemies... and from here the path to an autoimmune disease is short, in which the body attacks itself, and no one comes out of this war well.
In short, a headache, and one that takes years to resolve.
But here a wonderful thing happened.
Pfizer and Moderna used the most innovative technologies in biology to review the structure of the virus and its genetic code - tasks that at the end of the twentieth century would have required years - and identified a specific site (the spike protein) on the surface of the virus that they believed antibodies would bind well to. They then identified the instructions in the genetic code of the virus thanks to which it could produce the same spike protein. And finally, they duplicated the same instructions so that they appeared in a short strand of RNA - a substance that is abundant in cells. The RNA was supposed to enter the cells and make them produce the spike protein and display it on their surface so that the immune system would learn to recognize it and develop resistance against it.
How long did this whole process take? Moderna claims that it took them two days in total[vi]. They probably exaggerate, but not by much. A process that would have taken years in the past - will be done in 2020 in just a few days.

Second phase: the pre-clinical phase
The pharmaceutical companies are reaching the second stage of the route with their vaccine, and want to do clinical trials with it on humans. But before they are even allowed to approach people with a syringe, the companies must test the substance on at least two species of animals to make sure it does not cause harm.
All the information from these experiments - called preclinical experiments (that is, "before the clinical") - goes to a committee that examines it carefully. This committee makes no concessions to anyone. If the company rounded corners, for example, and chose to conduct an experiment exclusively on male mice, even though there is a fear that the substance being tested may cause developmental delays in the fetus, the committee will throw the researchers from all levels back to the laboratory, and demand that they show it experiments on females as well, or on animals that are particularly vulnerable to substances of this type this.
The preclinical phase can easily take a year or two. So how did Pfizer and Moderna manage to complete it at record speed?
Here we return to the companies' choice to use RNA vaccines. RNA breaks down quickly in the body, so there is no fear of serious side effects. There were also plenty of experiments in laboratories in the past with RNA vaccines, which did not show that it had a negative effect on animals.
But the vaccine does not consist only of RNA. The RNA is the final message we send to the cells, but in order for it to get there safely, it needs a 'truck' to bring it to its destination. This truck is a casing made of a material known as PEG (Polyethylene Glycol for my former students from the Faculty of Biomedical Engineering, who today already know much more than I do in these fields, and no, I'm not bitter at all. About that). Also regarding this substance, there are plenty of studies from the past that show that it is not toxic to the body in low doses. So the standards for the preclinical trials were not high to begin with, because the toxicity of all the relevant substances is known.
Pfizer specifically performed experiments on mice and macaque monkeys, tested the effect of the vaccine on them for 28 days, and when no signs of damage were detected - it received permission to proceed to clinical trials[vii]. I admit that I would like to see results from longer experiments on animals - two months or even more - but I am also willing to compromise on what is available, if the experts from the FDA committee decided that this is a long enough period of time to be sure *enough* of the safety of the vaccine. This decision must have had something to do, again, with the fact that both RNA and PEG vaccines are already well recognized as safe from the last ten years.
Third step: Submitting a request to open the clinical trials
At this stage, the companies submit the IND - Investigational New Drug application to the FDA. They describe the experiments they conducted, the chemical and biological structure of the tested substance, the way they will perform the clinical trials, the production methods of the vaccine and much more. All in all, it is an encyclopedia-sized amount of documents, and the FDA approves or rejects the continuation of trials within thirty days[viii]. I'm guessing that in the case of the corona virus vaccines, the FDA ordered several dozen employees to drop everything and concentrate on the information submitted by Pfizer and Moderna to approve it in a matter of days.
Stage Four: The Great Split
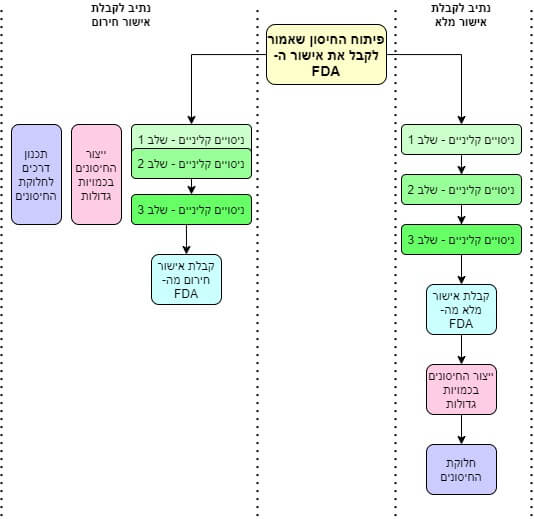
Here there is a step that is very different between the two types of approvals - the normal approval and the quick approval. Normally, the companies must carry out the clinical trials and only then can they get approval (BLA) to produce the vaccines in large quantities[ix]. In an emergency situation, the companies receive special permission to start preparations for the production of the vaccines already during the clinical trials. Thanks to this approval, they were able to open factories and adapt special production lines for the benefit of the new vaccine... even before it was even approved by the FDA. This shortcut saved them at least a year, and thanks to it they were able to start producing the vaccine even before the end of 2020.
Fifth stage: the clinical trials
And here, finally, we have reached the clinical trials. There are three phases of clinical trials:
- First step: an experiment on dozens of completely healthy volunteers, designed only to check that the vaccine does not harm them and to determine the optimal dose for them.
- Second step: an experiment on hundreds of volunteers, designed to test whether the vaccine is effective.
- Third step: an experiment with tens of thousands of volunteers designed to finally verify that the vaccine is effective and safe.
Pfizer chose to combine the first and second phase of the clinical trials. This is not an unusual procedure. On the contrary: many companies use it. Moderna was satisfied with the first stage only. Both tested only 45 volunteers in the initial clinical trials. They released the results in mid-July (Moderna)[X] and in mid-August (Pfizer)[xi]. In short: everything is fine.
And again, at this point the companies would have had to submit the results to the FDA for approval on the usual days, and the whole process would have taken between six months and a year. But in a plague as in a plague, the FDA pushed the matter to the top of its priorities, and in a very short time the approval was received to move to the third stage.
In the third stage, tens of thousands of vaccinators were tested. Collecting such a quantity of participants in experiments takes very long months and costs a fortune. This time, people flocked to participate in these experiments of their own free will. Another shortcut made possible by the situation. Pfizer and Moderna vaccinated about half of the participants, then released them into the wild and followed them for ten weeks (two and a half months) to see who got infected and who didn't.
Think about it for a moment: with regular vaccines - say, against polio or herpes - you have to wait until your recipients become infected with these diseases. It could take years. But when there's an epidemic out there, with a particularly contagious virus? In those ten weeks alone, 170 participants in the Pfizer trial were infected, and 196 participants in the Moderna trial. Almost all those infected came from the control group, while the vaccinated were almost never infected, and those who were infected - only mildly ill [xii] [xiii].
And what about the possible side effects? Well, there is a reason for the ten-week follow-up of the vaccinated. Most of the side effects of vaccines appear up to the sixth week from the moment the vaccine is given. And despite this, during the ten weeks no serious side effects of the vaccine were discovered. At most, an allergic reaction appeared in 0.63 percent of the vaccine recipients... and in 0.51% of the placebo recipients (that is, an injection without a vaccine). Today, after tens of millions of vaccine doses have been administered worldwide, it is still impossible to find severe side effects with high frequency[xiv]. There are certain suspicions that the vaccine may cause heart disease, but these have not been proven and are still being investigated. Even if this is true - it is probably a prevalence of one out of every hundred-thousand vaccinated people, approximately. And since without the vaccine we would all be infected with the British variant, and since the chance of getting a serious disease is much higher than one in a hundred thousand, approval of the vaccine is still justified.
And that's the end of the story: the results from the trials were so clear, and the side effects of the vaccines were so mild, that the FDA quickly granted emergency approval. And since the vaccine factories had already gone into action at the very beginning of the clinical trials, as soon as the final approval came, millions of vaccine doses were immediately sent to the early orderers. You may have noticed that here in Israel we received quite a lot of them.
Correction of an error regarding the fourth phase of the clinical trials
There are those who mention the "fourth stage" in clinical trials, and claim that we should have delivered the vaccines through it. This is a misunderstanding. The fourth stage is also known as the "post-marketing control" stage[xv]. That is, the company and the FDA continue to collect data about the vaccinated, to make sure that no problematic side effects emerge. The assumption after the third stage is that the vaccine is safe, but just to be sure - we continue to monitor all the vaccinated. Even if the vaccine had received normal approval, the fourth stage would have started only after receiving the approval.
And yet - why an emergency permit?
You must have noticed that I am optimistic about vaccines. They went through all the steps required to get the emergency clearance, after all. These steps were similar to those that vaccinations go through in a normal situation to get a normal approval. So yes - I have full confidence in the effectiveness and safety of the vaccine in the short term.
But in the long run, you can never really be sure. And this is the real problem: the experiments in the third phase were relatively short, because they showed such good results. This is a blessing, but it also makes it difficult for us to ensure that vaccines do not have long-term side effects. So it's true - they shouldn't have long-term side effects. But in biology you can never know with one hundred percent certainty what exactly is going to happen. At most it can be said that - "according to everything we know about the vaccine, there is no reason for it to have side effects in the long term".
Didn't calm you down? I totally understand. As I said to anyone who asked me privately: I would very much like to see long-term clinical trials for this vaccine, just to be safe.
What else? Now it is already clear that those who do not get vaccinated, will be infected. And if I get infected, my chances of going to the hospital are tens of times higher than the chances of side effects in the short term. And unlike the vaccine - we already know that those who are infected often develop side effects that accompany them for many months, and perhaps beyond that.
In other words, it is about simple risk management: on the one hand there is the virus, which we know can cause severe damage to the body, and the chances of contracting it are very high. On the other hand, there is the vaccine, which we know for sure is not harmful in the short term, and which we have no reason to think is harmful in the long term.
In such a situation, I prefer the vaccine, even though it was 'only' emergency approved.
And this is the whole story behind the claims regarding the limited emergency authorization. Yes, it is really limited, but the vaccines have gone through all the test steps that are also required for a normal approval. They just went through them very, very quickly, and it's a very good thing. The long-term risk is there, but it is minimal.
I have already received the vaccine, and I do not regret it. The emergency permit is absolutely sufficient here.
[I] https://www.fda.gov/news-events/approvals-fda-regulated-products/about-fda-product-approval
[ii] https://www.qualio.com/blog/fda-medical-device-approval-process
[iii] https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
[iv] https://www.historyofvaccines.org/content/articles/vaccine-development-testing-and-regulation
[v] https://www.visualcapitalist.com/the-race-to-save-lives-comparing-vaccine-development-timelines/
[vi] https://www.businessinsider.com/how-moderna-developed-coronavirus-vaccine-record-time-2020-11
[vii] https://www.biorxiv.org/content/10.1101/2020.09.08.280818v1.full.pdf
[viii] https://www.fda.gov/drugs/investigational-new-drug-ind-application/ind-application-procedures-overview
[ix] https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/vaccine-development-101
[X] https://www.nejm.org/doi/full/10.1056/NEJMoa2022483
[xi] https://www.nature.com/articles/s41586-020-2639-4
[xii] https://www.sciencemag.org/news/2020/11/absolutely-remarkable-no-one-who-got-modernas-vaccine-trial-developed-severe-covid-19
[xiii] https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
[xiv] https://medshadow.org/covid19-vaccine-side-effects/
[xv] https://www.news-medical.net/health/What-is-a-Phase-4-Clinical-Trial.aspx
More of the topic in Hayadan:
