What is spin? And where did the idea come from? This is a story about small steps, witty guesses and a struggle against giants
If you ask physics students what spin is, it is not certain that everyone will know the answer. Not because they have never heard of it, but because the intuition for spin in quantum mechanics is not the same as its classical counterpart. The story of spin began at the beginning of the 20th century when evidence accumulated that atoms with an even number of electrons are more chemically stable than those containing an odd number of electrons. In 1919 Irving Langmuir argued that the periodic table could explain chemical stability as long as the electrons were bound or grouped in a way that was not clear at the time. The chemical properties are based on the structure of the atom which was still in its infancy but in 1920 the picture began to become clearer. Niels Bohr, one of the pioneers of quantum theory, proposed the idea that electrons orbit the atomic nucleus in discrete orbits. The idea was received sympathetically in the scientific community and for the first time predicted the quantum nature of the particles of matter. Despite the success, experiments in nuclear physics indicated twice the number of possible states in the atom for each electron contrary to theoretical predictions. This phenomenon was predicted in the famous experiment of Otto Stern and Walter Gerlach in 1922. The physicists passed silver atoms (electrically neutral) through a changing magnetic field and observed how the silver beam split into two separate beams, one moving upwards and the other downwards. At that time the experiment was attributed as proof of the discrete nature predicted by Bohr and Sommerfeld, but in the future it will be recognized as an experiment that proved the existence of the quantum spin. To understand Stern Gerlach's experiment, you need to know some scientific facts: electrons moving in circles generate a magnetic field with a strength that depends on the electron's trajectory. The resulting magnetic field reacts to external magnetic fields and in their presence a force is exerted on the particles. Because in the Stern Gerlach experiment the field is not uniform, the force acting on the particles is not zero and as a result it deflects their trajectory - the greater the external field or the field created on the particles, the greater the deflection. From this point on, quantum mechanics plays a significant role - due to the fact that the electron moves around the nucleus in defined orbits, the magnetic field produced from rotation around the nucleus is also quantized (or discrete). If discrete trajectories exist, then the silver particles will only be deflected along defined trajectories, again depending on the strength of the field they produce. The split in the experiment is created because the electron can rotate clockwise or counterclockwise. Of course, there is a problem with this interpretation: we know that electrons do not really circle around the nucleus, but are described as a probability cloud in shapes that really do not resemble circles (at least not those at the lowest energy level). In the end, we mixed classical intuition and observations from quantum mechanics, so the idea is not really cooked (a more in-depth interpretation will be provided later in the article). By the way, the experiment was also conducted on an electron beam (without a nucleus) and the splitting was still predicted, even though the Lorentz force predicts for charged particles movement in one direction! How?
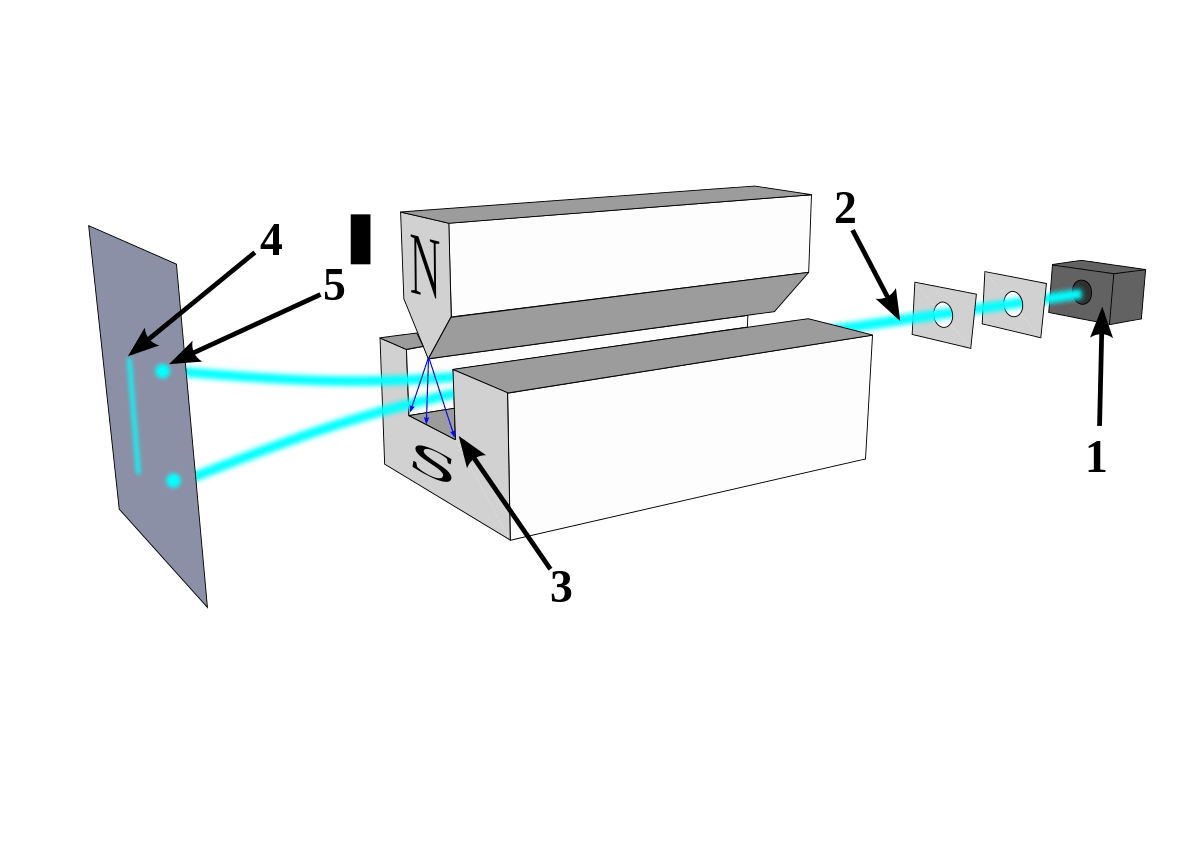
These experiments alongside the analysis of the light emitted by energetic atoms led Wolfgang Pauli to publish the famous prohibition principle in 1925 according to which two electrons cannot share the same quantum numbers (in other words, two electrons cannot be in the same physical state). The brilliant idea caught the attention of two physicists in the making - Ullenbeck and Godschmidt, doctoral students under the guidance of Paul Arnfest. The students thought of a simple way to explain the prohibition principle - electrons have internal spin, not from rotation around the nucleus but from rotation around their axis. They shared their thoughts with Ehrenfest who decided to send a letter to Lorenz to discuss the "witty idea" with him. Lorentz quickly dismissed the idea, arguing that spin for an electron was incompatible with classical electromagnetic theory.
Lorenz had good reason not to believe the young guys. To produce the electron's magnetic field and angular momentum, and given the upper limit to its size, the internal rotation must be faster than the speed of light. Following Lorenz's response, Arnfest's doctoral student begged not to publish the idea, but it was already too late. Ehrenfest tried to calm his students by claiming that "you are young enough to afford a stupid idea!". It turns out that a few months before, another doctoral student from Columbia University thought of the same idea and decided to send Pauli a letter for reference. Pauli thought the idea was ridiculous and in response to the student he said: "This is indeed a brilliant idea but it has nothing to do with reality." The PhD did not publish the idea and did not receive the credit for fear of publishing a mistake. Not for nothing did Ollenbeck say that he was lucky to have been a student of Ehrenfest and not of Pauli. Fortunately for them, Bohr supported the original idea and soon the scientific community aligned with him. In a relatively short time, several papers used the electron spin to explain the formation of white dwarfs, the behavior of electrons in metals and the complete theory of quantum electrodynamics.
After Scholl's experiment that performed the Stern Gerlach experiment on electrons, there was no doubt about the existence of the electronic spin. Experiments in the XNUMXs and later indicated more particles with spin, similar to the electron, and the term was no longer limited to a single particle. So what is spin? If the classical explanation is insufficient, quantum mechanics must provide an answer. Let's start with the name, why spin? The name draws inspiration from classical electrodynamics - an external magnetic field uniquely affects the movement of an electron moving in a circular orbit (with angular momentum). In the Stern Gerlach experiment, the electrons react similarly to particles with angular momentum, but they do not rotate around a nucleus, so the natural explanation is that electrons rotate around themselves. Classically we saw that this idea contradicts private relativity and therefore the analogy is not accurate. Despite the controversy over the classical interpretation, this does not mean that similar quantum phenomena cannot be observed with classical particles rotating around themselves. In the article Published a few days ago in the prestigious magazine Nature, researchers from the United States, France and Sweden demonstrated how bouncing droplets can behave similarly to particles with spin. The researchers arranged the drops in a helical array and allowed them to rotate clockwise or counterclockwise. The lattice structure is supposed to resemble crystals and the particles inside the droplets. Every time the drop bounced across the surface of the water it created a ripple underneath that its neighbors could feel. The experiment showed that the bouncing particles react to the ripples around them and change their internal rotation. Similar to magnetic materials that create regions with the same spin (that is, with the same internal rotation - clockwise or counterclockwise), the drops also tended to arrange themselves according to their neighbors. Under certain conditions the researchers were able to make all the droplets line up in the same direction. Directionality is important in magnetic materials - if the spin is like an internal magnet (similar to a particle rotating around a nucleus creating a magnetic field), then the more electrons there are arranged in the same direction, the greater the magnetic field in the material. Such synchronization appears in all kinds of systems in nature, even in biology. As darkness falls, fireflies flash at different frequencies, but as night falls, the frequency of flashes in the swarm synchronizes.
In the XNUMXs a small community of researchers nevertheless tried to describe the spin in classical terms (a modern text is cited HERE). According to the field theory, the electron is described by a field and therefore does not have to define a finite size for it. The researchers claimed that a circular flow of the electronic field applied to the entire space can create the same spin properties for the particles of nature and in particular for the electron. Despite the fascinating idea, modern physics treats spin in a different way - it is simply another charge carried by the particles in nature, similar to electric charge or angular momentum. According to quantum mechanics, the internal charges arise from symmetry that manifests itself in nature. Angular momentum or spin results from rotational symmetry of either spatial axes or internal axes in the particle's fields. The same goes for the electric charge - the electromagnetic field does not change under calibration symmetry. This symmetry is associated with the conservation of electric charge. The relationship between conservation laws and symmetry requires its own chapter, but to summarize things in quantum mechanics, classical thought is broken and intuition is built based on mathematics that is free from prejudice and human perception. In quantum mechanics, particles are at most objects with measurable quantum numbers - energy, angular momentum, mass, electric charge/color and spin. These quantum numbers affect the dynamics of the particle and in particular the spin affects the behavior of particles in the presence of an external magnetic field or other particles with spin.
Each week I will dedicate an article to an idea or a common concept in modern physics. If you have suggestions or requests for this corner, you are welcome to contact me at the email address: Noamphysics@gmail.com
More of the topic in Hayadan:
- Basic concepts in quantum physics: wave-particle duality
- A twist on the paradox of information disappearing inside black holes
- A device that will make it possible to answer one of the central questions in physics that remain unanswered
- A new state of aggregation in a two-dimensional material has been discovered
- Researchers were able to write multi-state magnetic memory using spin currents


13 תגובות
Stephen Hawking beautifully described what spin is in "A Brief History of Time". Since it is not really a rotation on an internal axis, the definition is how the particle "looks" from different angles. The problem is that this explanation is not the most intuitive either
https://sayitwithscience.tumblr.com/post/9182647836/the-mention-of-spin-of-a-particle-is-one-that
Nisimov Anonino Inal Darbacom, where did you go? Nice, are you still near the pole?
The point is that the electromagnetic radiation from an accelerated charge can be measured regardless of the speed of the measuring device relative to the charge.
So who was right? Newton who said that a body in free fall accelerates, and is not accelerating when it is placed on the table at rest, or Einstein who claimed the opposite? What will the measurements show?
In the past, it was argued here that the principle of the equivalence of gravity and acceleration is not comprehensive, but no detail was ever given. Maybe Noam can explain?
And why does no one address my question in the article about duality:
If we have two fully entwined electrons in the room, and one of them is measured at 2 and the other at 4, can we say that before the first was measured at 2 it did not have a quantum state (spin for example) and it was the measurement that determined its state? On the other hand, the second measured at 4 must have the opposite state which we know long before the measurement? Therefore we can say that the measurement of the first determined the quantum state of the second, but not the other way around.
It is said that we have two spaceships passing each other and in each of them a particle is intertwined with a particle in the other spaceship. At the moment of the suit, the clocks in the spacecraft show 0. In the first, the particle is measured by 3 times six times, and in the second by 5.
Will the spins be reversed in the particles?
Who can say that the first measurement at 3 determined the second measurement at 5 but not the other way around?
Miracles
Do not know..
I asked Wikipedia,
She says there is no such thing as a *stationary electron*...
I asked you to check again - you started arguing with me..
Hopefully next time I will have the opportunity to enter it again...
In the meantime, I sent a letter of apology (with a flower), but she hasn't gotten back to me yet...
Oh well..
Not everything in life goes smoothly...
What I wanted to say..:
It is clear that a stationary electron (or dead or virtual or non-binary) does not radiate - because it does not exist.
Those that do exist (a standard or normative electron or one that points to white-male) radiate and how! The fact that an atom emits energy means that you can perceive this energy with your senses.
You can also "catch" the emitted energy with dedicated devices...
Successfully.
Good Day.
anonymous
Stationary electrons do not radiate. The "field" is the result of simulated photons - you will not detect radiation from such a charge.
If a falling electron radiated then it would fall more slowly than another particle that has no charge.
It has a detailed explanation on Wikipedia.
Miracles
Why does a "charge in a gravitational field not radiate"?
Again, if the charge does not radiate then it does not exist.
You, in fact, support my words and reinforce them.
The second part of your response is not clear.
good week.
anonymous
A charge in a gravitational field does not radiate.
More than that - if you drop an electron and a neutron in a gravitational field - they will fall together.
we
I place my radiation meter 20 meters away from the table on which an electrically charged body (a block of rubbed amber) rests.
What is the radiation strength I will measure? the wavelength? The polarization?
And if I put 2 bodies on the table that are identical to the first one - will the radiation be measured twice as strong?
Our Israelis
An electric charger placed on the table does radiate.
You are also in relativistic acceleration.
The radiation that comes out of the charge placed on the table is what makes you feel the charge itself.
If the charge was not radiant, you would not have felt the charge on the table and you would not have known that there was a charge on the table.
...it's all radiation, the rest is history))
An electric charge radiates with acceleration.
Electrical radiation can be measured.
What happens to a charged body in free fall?
According to Newton - it is accelerating.
According to Einstein - no.
So will it be broadcast or not?
What's more: according to Einstein's principle of equivalence, acceleration and gravity are equivalent.
Therefore, according to Einstein, a body lying on the table in the living room at rest is actually in acceleration.
So why does an electric charge placed on the table not emit measurable radiation? Or maybe he is?
In short - bottom line, we don't physically know what spin is, we accept the fact that it exists and can be measured, excellent article
https://davidson-weizmann-ac-il.cdn.ampproject.org/v/s/davidson.weizmann.ac.il/online/maagarmada/physics/%D7%9E%D7%A7%D7%95%D7%A8-%D7%9E%D7%AA%D7%97-%D7%97%D7%93%D7%A9-%D7%9C%D7%96%D7%A8%D7%9E%D7%99-%D7%90%D7%9C%D7%A7%D7%98%D7%A8%D7%95%D7%A0%D7%99%D7%9D-%D7%9E%D7%A7%D7%95%D7%98%D7%91%D7%99%D7%9D?amp_js_v=a6&_gsa=1&&usqp=mq331AQKKAFQArABIIACAw%3D%3D#aoh=16289256808617&referrer=https%3A%2F%2Fwww.google.com&_tf=From%20%251%24s&share=https%3A%2F%2Fdavidson.weizmann.ac.il%2Fonline%2Fmaagarmada%2Fphysics%2F%25D7%259E%25D7%25A7%25D7%2595%25D7%25A8-%25D7%259E%25D7%25AA%25D7%2597-%25D7%2597%25D7%2593%25D7%25A9-%25D7%259C%25D7%2596%25D7%25A8%25D7%259E%25D7%2599-%25D7%2590%25D7%259C%25D7%25A7%25D7%2598%25D7%25A8%25D7%2595%25D7%25A0%25D7%2599%25D7%259D-%25D7%259E%25D7%25A7%25D7%2595%25D7%2598%25D7%2591%25D7%2599%25D7%259D
An eye-opening article. Clear explanation and enjoyable reading.
A. If an electron is near the nucleus in a 'probabilistic' position and not in one fixed position, then it can be in several energy levels depending on its distance from the nucleus, and therefore the 'quanta' is not completely univalent.
B. The atoms do not change. Those atoms have existed on Earth since they were formed, let's say 5 billion years. Where does the energy come from for the 'spin' that has been going on forever for 5 billion years?
magnificent. A very successful project. It is hard to believe that you can understand spin through an article. In any case, it requires a great deal of prior knowledge and even then, it is likely that the reader remains in the imagination with an image of an angular tena and leave it to him. You need to find and vary as many analogies as possible in the language in order to demonstrate spin - for example the spinning ball, for example a property of a material, for example the direction of the field it creates. Great project. Looking forward to learning more!!!
Thank you!!!!