Where is the quantum in quantum mechanics? It turns out that nature is not discrete at all, as long as there is nothing to force it to be so
Quantum physics raises many associations. Usually, those who are not knowledgeable in the field will assume that this is a discrete description of nature. Phrases like "everything comes in portions", "quantums = particles?", "the universe is discrete and not continuous", and also "particles are waves and vice versa" have become canonical explanations that cause confusion and discomfort. It is true that there are sizes that are limited to have discrete values, but in fact, if you look at the equations that dictate the dynamics of the particles, you don't see any sign of the discrete nature of the universe. What does the last sentence mean? Quantum mechanics as a whole is based on the wave function which gathers the total probabilities of being in any state in nature (in other words, the wave function is simply a simple sum of states, for example a*state1+b*state2 when the coefficients a,b in absolute value squared will describe the probability of being in the state ). The wave function evolves in time according to the Schrödinger equation. If we recall the equation (an expansion on the Schrödinger equation can be found in the previous article) appears to be completely continuous because a derivative requires a smooth space (in location and time). From this equation we cannot know in advance whether the wave function describes a discrete number or a continuous space of possibilities. Still, there is no lack of examples that measurable quantities in nature are limited to discrete values. For example, the "energy levels" or the possible paths of the electron associated with the atomic nucleus are not continuous parameters, meaning they can be enumerated and are separated by a finite distance from each other. Where then did the discrete universe suddenly come from?
Everything starts and ends with waves. First, notice the concept of "bound electron" that we mentioned before. If the electron is completely free, i.e. no forces act on it, there is no reason for its energy to appear in discrete portions, or in other words there is no reason for the electron to move only in limited orbits. The fact that the electron has allowed and forbidden paths is mainly due to some kind of constraint. Because the mathematical formulas of the wave function are very similar to wave mechanics, we will try to imagine a classical wave system that would be equivalent to the quantum one. Given a string tied at its ends, standing waves of all kinds of frequencies can be excited in it.
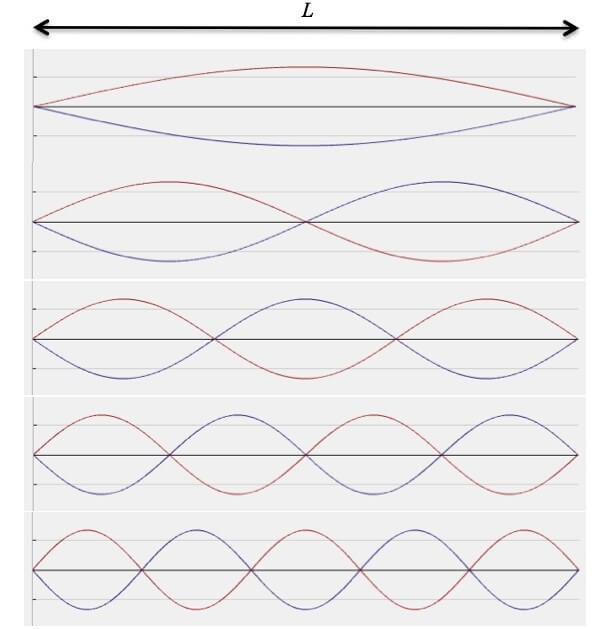
A standing wave from our point of view will describe a particle with a defined energy, because for a standing wave a unique frequency can be defined and each frequency defines an energy for the particle from the principle of duality (Wave-particle duality). But if we conduct the experiment on such ideal systems it seems that we are not able to excite any frequency we want on the string. The permitted frequencies will be:
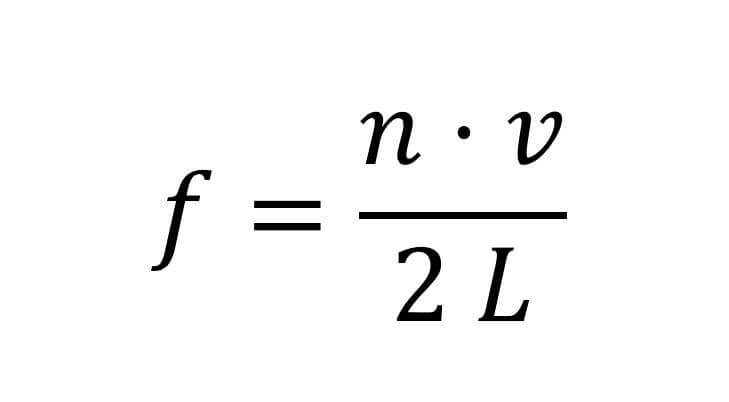
Where L is the length of the string, v is the speed of the wave and n- is an integer that can have the values 1,2,3...
The fact that the frequency f is obtained as integer multiples of a magnitude that depends on the length of the string means that it is not possible to create any frequency that we want. The reason why we got allowed frequencies is due to the constraint we added to the string - we grabbed it from both ends and forced the wave at the end point to zero. We will now try to move to a quantum system. Let's imagine a particle in the hole blocked by a very high wall (to be precise, a wall of infinite height and infinite thickness). If we neglect the small tunneling effect (which does not exist provided the wall is infinite), we prevent the particle from being inside the wall and behind it. In other words, the probability of the particle being in this region is zero. Mathematically, the experimental observation we have just deduced translates to work that the particle's wave function zeroes out at the edges, just like a tied string.
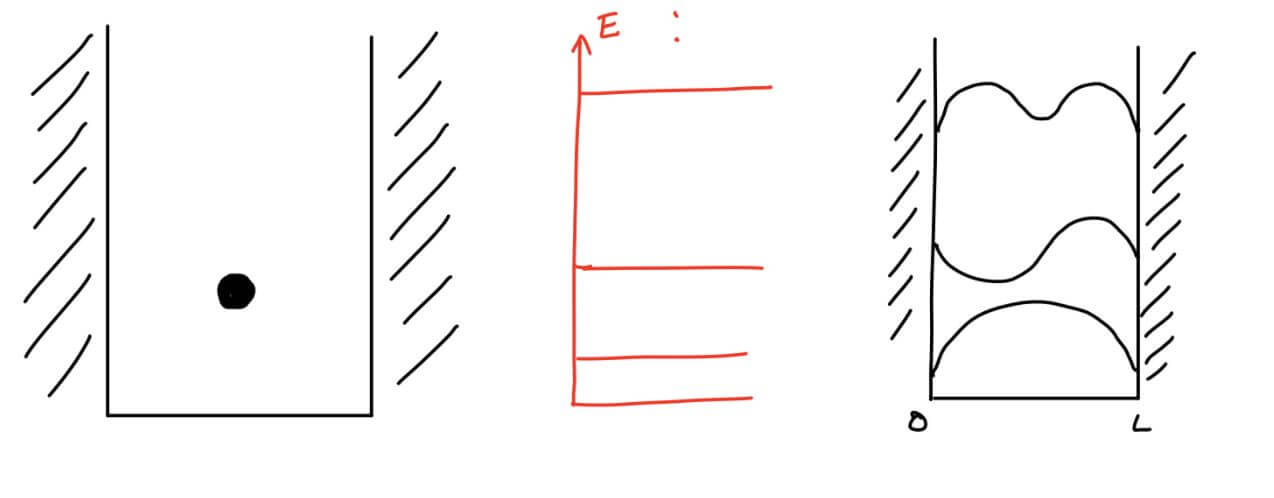
The deep pit is actually the potential energy that the particle feels in its environment. The electric force that pulls the electron to the center of the nucleus can also be described as a hole. The following drawing shows, for example, the effective energy felt by the electron under the influence of a single proton in the nucleus (vertical axis) as a function of distance (horizontal axis).
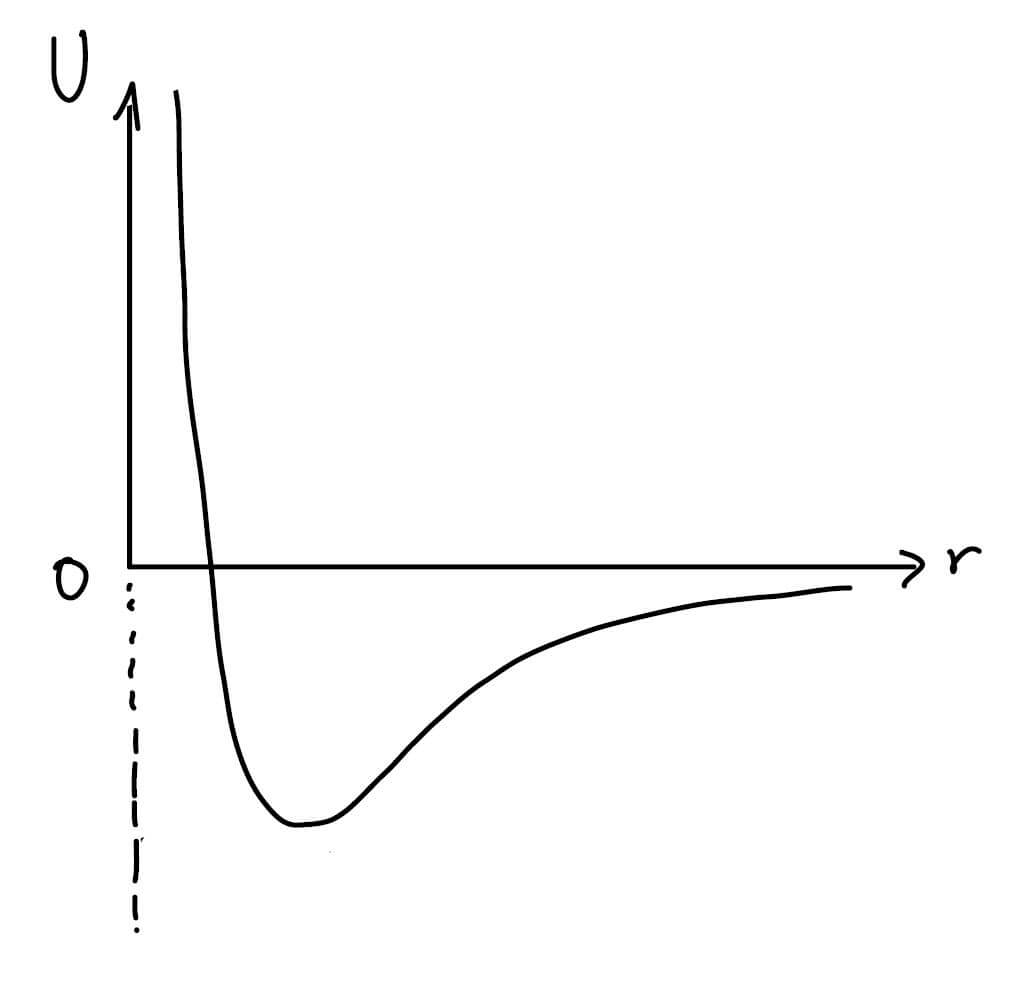
If we think of the electron as a ball rolling on a surface whose edge is fixed by the graph, the electron will tend to fall into the valley. Physicists call the bottom of the valley the fundamental level because this is the point that the electron will strive to be at the lowest energy. It is important to mention that the uncertainty principle forbids the particle to be located precisely (otherwise the uncertainty in momentum is infinite and at the level of the principle there is a probability of finding a particle with infinite energy as well, and this is a forbidden physical state). Therefore, one should be careful in the argument that the particle is indeed at the very bottom. At the same time, the uncertainty has a significant contribution to the behavior of the effective energy at small radii - in this area the electron is concentrated around the nucleus and therefore the uncertainty in the position is very small. As a counter-reaction, the uncertainty in the momentum increases and the effective energy increases to infinity. We note that the divergence (the significant increase to large values) that appears in the effective energy for small radii can also result from the angular momentum (assuming it is not zero). Now let's recall the fact that the electron is "bound": the fact that the particle is inside the hole means that it does not have enough energy to escape from it. Classically we will need to lift the particle above the hole to get it out of the trapped state, but quantumly as long as the barrier is at a finite height it can be tunnelled, the question is where? In the upper video it appears that for particles inside the hole (with negative energy) the wall continues forever, therefore the tunnel has no real meaning. The mathematical conclusion is that the wave function at large radii must zero because the probability of finding the particle there is zero. Does this sound familiar to you? Of course it is, this example is very similar to a tied string or a particle in a flat pit. It is therefore observed that the energies allowed for the electron will be discrete up to a certain threshold, determined by the height of the hole, which dictates the energy required for the electron to detach from the atom.
The obvious conclusion from these examples is that the discrete nature of quantum mechanics arises from a constraint set by the system and is not a rule of nature. One necessary comment is regarding the common argument that "the light comes in portions". Pay attention to the poet's intention - it is not that the energy spectrum of the photon (particle of light) is discrete, the light particle can carry any energy, but rather the fact that in some cases the energy of light can be regarded as carried on a single particle and not on a continuous object in space like a wave. A final note regarding the Schrödinger equation - beyond the fact that the mathematical description of quantum mechanics does not at all hint at one or another discrete nature, it also does not at all hint at its probabilistic nature. The Schrödinger equation is completely deterministic, there is no random element in it. The randomness is part of the assumptions of quantum mechanics that derives from experimental observations.
Each week I will dedicate an article to an idea or a common concept in modern physics. If you have suggestions or requests for this corner, you are welcome to contact me at the email address: Noamphysics@gmail.com
For those interested, I attach as an appendix a sketch for a short calculation of why the frequency on a tied string is discrete. A similar calculation is also done for a particle in a pit with infinite walls. I will point out that the calculation is done for zero time, but it is correct for every moment.

More on the subject On the science website:

7 תגובות
Very interesting
It is stated in the article: "The discrete nature of quantum mechanics arises from a constraint set by the system and is not a rule of nature."
What does it mean that the compulsion is not nature?
Zen says the compulsion is a divine miracle?
"Nofach" pushed-destroyed "Nofach"...
You should also know Hebrew.
Nice article but still confusing. Discrete in the physical world is not mathematically discrete. The mathematical equations are merely a symbolic model of the laws of nature. Most of them are correct only in a limited area and because of this, Einstein's theory of gravity, for example, computationally improved Newton's theory of gravity. The discrete model that derives from the mathematical wave theory is indeed not a proof of the singularity of space and perhaps time as well. As noted at the end, indeed only the experiment can confirm the real loneliness in the universe. As Kroenker said something like this: "God created the integers/discrete numbers, everything else is the imagination of the people" - in the original: "God created the integers, all else is the work of man"
Thanks Elon for the clarification. You're right, I didn't expand enough on the matter and your comment adds more volume to this sentence.
"One comment is required regarding the common argument that "the light comes in portions". ... It is about the fact that in some cases the energy of light can be considered as carried on a single particle and not on a continuous object in space like a wave."
Not accurate.
It is about the fact that the *'height' of the wave*, whether the wave itself is spread over infinite or finite space, can only have discrete values, meaning that the *amount of light* is an integer multiple of some basic quantity. This basic quantity is called a "photon".
The reason for this is that when you write down the energy equation of an electromagnetic field, you get a square pattern in the electric and magnetic fields. This pattern is the same as that of particle energies in a harmonic oscillator, where the momentum and position are squared. Therefore, just as the energy of a quantum particle in a harmonic oscillator is discrete (this is another example similar to what you described in the post), so also the total energy of an electromagnetic field, that is, the 'height' of the electromagnetic wave, can only have discrete values.
Wubernium also behaves in quantum physics
Wakanda and Canada wakanda